2. 中国水产科学研究院长江水产研究所, 湖北 武汉 430223
2. Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences, Wuhan 430223, China
雌激素是动物性别分化与性腺发育的重要调控因素, 其功能发挥可通过核雌激素受体(Estrogen receptors, Esrs)介导的基因组效应, 也可通过G蛋白偶联雌激素受体(G protein coupled estrogen receptor, Gper)介导的非基因组效应快速激活细胞内信号通路[1-4]。雌激素通过基因组途径发挥功能需较长时间, 而Gper介导非基因组途径能快速响应雌激素并激活Mapk/Erk通路[3], 弥补了Esrs功能的不足。Gper是一种膜雌激素受体, 属于膜受体Gpcr亚家族。该蛋白家族具有7个跨膜α双螺旋结构域, 参与多种信号通路, 响应内外环境对机体的影响[5-6]。Gper参与调控动物性腺发育[7]、免疫功能[8]、神经系统与骨骼功能[9]等多方面生理作用, 是雌激素作用的重要途径[1, 10]。
已有研究表明, Gper在动物性别分化与性腺发育中有重要作用。Ge等[11]发现在原鸡(Gallus gallus)原始生殖细胞中Gper表达时间早于Esrs, 且雌激素通过Gper/Egfr/Akt/β-catenin级联反应调控早期生殖细胞增殖; Wang等[12]在雌性仓鼠(Cricetidae)中发现干扰Gper的表达会抑制原始卵泡的形成; Pang等[13-14]研究表明在细须石首鱼(Micropogonias undulatus)和斑马鱼(Danio rerio)卵黄发育时期, 雌激素通过激活Gper阻止卵母细胞早熟。雄性动物中, Gper在生精细胞以及间质细胞、支持细胞都有表达[15], 对小鼠精原细胞的增殖具有促进作用[16]; Gper在欧洲鳗鲡(Anguilla anguilla)精子发生后期表达显著升高[17]; 分离斑马鱼早期和晚期生殖细胞发现, Gper主要在早期生殖细胞包括精原细胞、精母细胞中表达[15], 说明Gper参与雄性动物生殖过程并存在物种差异, 但其相关作用机制尚不明确。
中华鳖(Pelodiscus sinensis)属爬行纲(Reptilia)、龟鳖目(Chelonia)、鳖科(Trionychidae)、中华鳖属(Pelodiscus)。其肉质鲜美、营养丰富, 是中国重要的淡水养殖对象之一[18]。由于雄鳖有较快的生长速度、更高的经济价值, 因此研究其性别分化及生殖有助于中华鳖新品种的培育。本研究以中华鳖为实验材料, 利用RACE技术克隆得到Gper cDNA全长序列, 应用qRT-PCR技术对Gper mRNA在不同组织及胚胎发育时期的表达进行了研究。此外, 利用腹腔注射的方式对雄鳖注射来曲唑(Letrozole)和Gper抑制剂G-15, 研究Gper在精巢中的作用, 为进一步探索雌激素及其受体Gper的生理功能提供参考。
1 材料与方法 1.1 实验材料中华鳖受精卵取自河南省固始县晨源水产养殖专业合作社, 收集当日所产受精卵后分别置于(27±0.5) ℃和(32±0.5) ℃恒温恒湿孵化箱中孵化。按照中华鳖胚胎发育图谱[19]收集32 ℃孵化的胚胎, 并收集27 ℃同一孵化时长的胚胎。分离不同发育时期胚胎的性腺; 另取体健康2龄雌雄中华鳖各5只, 取脑、卵巢、精巢、肠道、心脏、肌肉等组织, –80 ℃保存备用。使用TaKaRa RNAisoTM Plus (TaKaRa, 大连)提取总RNA。利用1%琼脂糖凝胶电泳检测RNA完整度、紫外分光光度计检测RNA浓度。
1.2 中华鳖Gper基因cDNA克隆采用PrimScriptTM Reverse transcriptase (TaKaRa, 大连)试剂盒合成中间片段cDNA, SMARTTM RACE cDNA Amplification Kit (Clontech)反转录合成的cDNA则作为基因3′和5′端序列扩增模板。根据中华鳖转录组中Gper (XM_006112842.3)序列片段设计正反向引物Gper-F和Gper-R (表 1), 以中间片段cDNA为模板扩增Gper cDNA部分序列, 并克隆测序。在获得序列基础上设计3′ RACE和5′ RACE的特异性引物(表 1), 进行3′和5′末端PCR扩增。PCR产物进行回收纯化, 经连接、转化后筛选阳性克隆并送上海生工生物有限公司测序。
|
|
表 1 所用引物序列 Tab.1 The sequences of primers used in this study |
序列采用DNAStar软件包中SeqMan将Gper中间片段、3′ RACE和5′ RACE所得序列进行拼接, 获得cDNA全长序列。使用ORF Finder (http://www.ncbi.nlm.nih.gov/gorf.html)确定开放阅读框。采用Compute pI/Mw tool (http:///www.expasy.org/tools/pi-tool.htm)计算蛋白等电点和分子质量; InterProScan software (http://www.ebi.ac.uk/Tools/pfa/iprscan5/)查找保守结构域; SignalP 3.0 Server (http://www.cbs.dtu.dk/services/SignalP/)查找信号肽; TMHMM 2.0 Server (http://www.cbs.dtu.dk/services/TMHMM-2.0/)预测跨膜结构域; MEGAX以邻接法构建系统进化树。
1.4 中华鳖Gper基因表达模式提取胚胎发育不同时期性腺和成鳖不同组织总RNA, 以总RNA为模板, 按照Prime Script RT reagent Kit with gDNA Eraser (TaKaRa, 大连)操作说明进行逆转录合成cDNA, 并将cDNA稀释10倍作为qRT-PCR模板。依据获得的Gper基因全长序列设计荧光定量引物Gper-RTF和Gper- RTR, 并以Gapdh为内参基因(表 1)。Prime ScriptTM RT Master Mix和进行qRT-PCR, 反应体系(20 μL): 2×SYBR Premix Ex TaqTM (TaKaRa, 大连)10 μL, 上、下游引物各0.5 μL, cDNA模板2 μL, ddH2O 7 μL; 反应程序: 94 ℃ 10 s, 60 ℃ 15 s, 72 ℃ 20 s, 40个循环。每个样本设置3个重复, 依据2–ΔΔCt法计算实验样本Gper相对表达量, 利用SPSS 20.0软件对数据进行单因素方差分析(one-way ANOVA), P < 0.05表示具有显著性差异, 结果用平均值±标准误(x±SE)表示。
1.5 Letrozole和G-15处理对成年中华鳖性腺的影响将大小均一的雄鳖(300~350 g)进行腹腔注射, 一部分进行Letrozole (索莱宝, 中国)处理, 另一部分用于G-15 (APEXBIO, 美国)处理。两组处理分别设置对照组、低浓度处理组和高浓度处理组(n=5)。将Letrozole和G-15分别溶于DMSO (索莱宝, 中国), –20 ℃保存, 注射前用生理盐水进行稀释, DMSO:生理盐水=1:19。Letrozole实验组低浓度注射剂量为5 mg/kg, 高浓度为10 mg/kg。G-15实验组低浓度注射剂量为0.5 mg/kg, 高浓度为1 mg/kg。对照组注射相同体积的溶剂。实验同时进行, 中华鳖每周注射一次, 共注射4次。处理结束后分别取中华鳖睾丸, –80 ℃保存。按照1.4方法提取总RNA, 反转录合成cDNA进行qRT-PCR。相关基因引物根据NCBI数据库中华鳖基因组序列设计(表 1)。
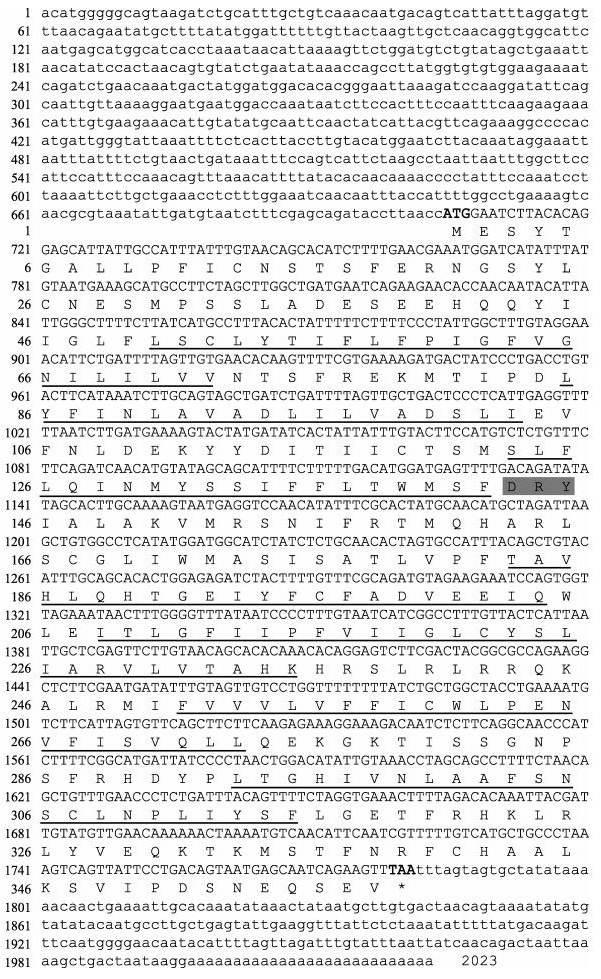
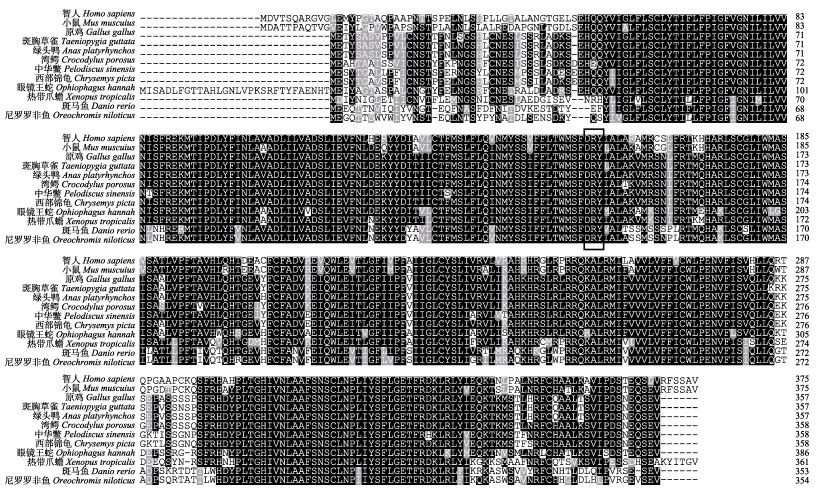
2 结果与分析 2.1 Gper基因全长cDNA序列特征和系统进化分析克隆片段经测序和拼接, 结果显示Gper cDNA序列全长2023 bp (GeneBank登录号: MK111424), 包括705 bp的5′-UTR, 1077 bp的开放阅读框和241 bp的3′-UTR, 共编码358个氨基酸残基, 相对分子质量为41.084 kD, 等电点为6.844。SignalP分析未发现有信号肽。THMHMM分析氨基酸序列发现Gper氨基酸序列含7个跨膜区(图 1), 多重氨基酸比对结果显示在第三跨膜区后存在视紫红质亚家族(A类)中保守的DRY (Asp-Arg-Tyr)结构(图 2)。
|
图 1 中华鳖Gper基因cDNA全长及氨基酸序列分析 小写字母表示3′和5′非编码区, 大写字母表示编码区, 加粗部分表示ORF区起始密码子(ATG)和终止密码子(TAA), 黑色下划线表示跨膜区, 灰色阴影部分表示DRY结构. Fig.1 Sequence analysis of full-length cDNA and amino acids of Gper in Pelodiscus sinensis The lowercase indicates 3′ UTR and 5′ UTR, and the coding sequence is presented in capital letters. The bold part represents the start codon (ATG) and the stop codon (TAA) in the ORF region; transmembrane regions are underlined by dotted line and the DRY structure is shaded in gray. |
|
图 2 中华鳖与其他物种Gper氨基酸序列多重比对 使用ClustalX进行多重序列比对, 黑色方框表示DRY三联体结构.绿头鸭(XP_012955573.1), 西部锦龟(XP_005289205.2), 湾鳄(XP_019397607.1), 斑马鱼(NP_001122195.1), 原鸡(XP_004945187.2), 智人(NP_001035055.1), 小鼠(NP_084047.2), 眼镜王蛇(ETE58208.1), 尼罗罗非鱼(XP_005468845.1), 中华鳖(XP_006112904.1), 斑胸草雀(XP_030141062.1), 热带爪蟾(NP_001107725.1). Fig.2 Alignment of Gper in Pelodiscus sinensis with those of other vertebrates The program ClustalX is used to align the Gper sequences. The DRY structure is boxed. Anas platyrhynchos (XP_012955573.1), Chrysemys picta bellii (XP_005289205.2), Crocodylus porosus (XP_019397607.1), Danio rerio (NP_001122195.1), Gallus gallus (XP_004945187.2), Homo sapiens (NP_001035055.1), Mus musculus (NP_084047.2), Ophiophagus hannah (ETE58208.1), Oreochromis niloticus (XP_005468845.1), Pelodiscus sinensis (XP_006112904.1), Taeniopygia guttata (XP_030141062.1), Xenopus tropicalis (NP_001107725.1). |
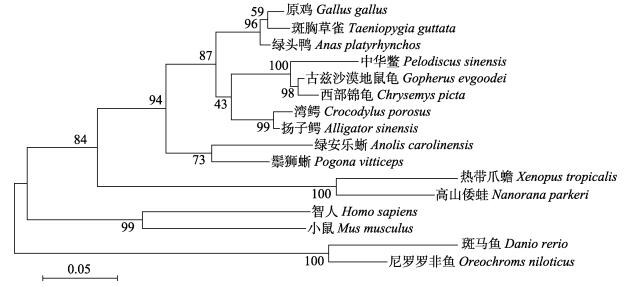
利用MEGAX构建系统进化树, Gper进化树可分为两支, 其中鱼类聚为一支, 鸟类、爬行类、哺乳类和两栖类动物聚为一支。中华鳖Gper与龟类亲缘关系最近, 其次是鸟类、爬行类, 这与中华鳖进化地位相一致(图 3)。
|
图 3 Gper系统进化树 扬子鳄(XP_006030771.1), 绿安乐蜥(XP_003225875.1), 古兹沙漠地鼠龟(XP_030435025.1), 高山倭蛙(XP_018425675.1), 鬃狮蜥(XP_020659978.1). Fig.3 Phylogenetic tree of Gper from vertebrates Alligator sinensis (XP_006030771.1), Anolis carolinensis (XP_003225875.1), Gopherus evgoodei (XP_030435025.1), Nanorana parkeri (XP_018425675.1), Pogona vitticeps (XP_020659978.1). |
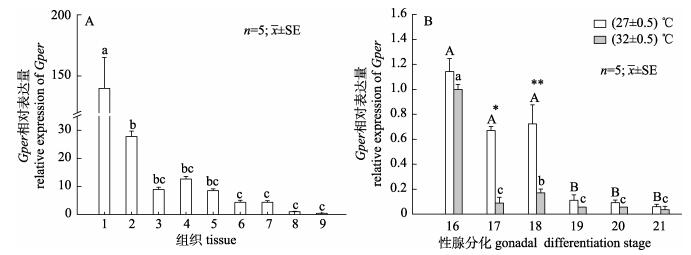
以Gapdh为内参基因, qRT-PCR结果显示, Gper mRNA在中华鳖各组织都有表达, 其在脑的相对表达量最高, 显著高于其他组织(P < 0.05), 其次是卵巢和脾脏。在精巢、胃、肝脏、肠、心脏、肌肉的表达量较低(图 4A)。检测不同孵化温度下性腺中Gper在性腺分化时期表达情况, 结果显示, Gper mRNA在两种不同孵化温度下表达呈现相似的变化趋势:在16期(性腺分化早期)的表达量最高, 随着性腺分化的进程, 表达量降低。27 ℃孵化条件下Gper表达量在19期显著下降(P < 0.05), 32 ℃孵化条件下Gper表达量在17期显著降低(P < 0.05)。27 ℃孵化温度条件下Gper的表达量比32 ℃时高, 并在17期和18期显著高于32 ℃时的Gper表达量(P < 0.05)(图 4B)。
|
图 4 Gper在成年中华鳖不同组织及胚胎性腺分化不同时期性腺中的表达 A. Gper在成年中华鳖不同组织表达分布; 1:脑; 2:卵巢; 3:精巢; 4:脾; 5:胃; 6:肝; 7:肠; 8:心; 9:肌肉. B. Gper在中华鳖胚胎性腺分化时期性腺中的表达; 16: 16期; 17: 17期; 18: 18期; 19: 19期; 20: 20期; 21: 21期. *和不同字母代表显著性差异(P < 0.05). Fig.4 The relative expression of Gper mRNA in different tissues and at different stages of gonadal in Pelodiscus sinensis. A. Tissues expression of adult Gper in Pelodiscus sinensis; 1: brain; 2: ovary; 3: testis; 4: spleen; 5: stomach; 6: liver; 7: intestines; 8: heart; 9: muscle. B. The relative expression of Gper mRNA in different stages of gonadal. 16: stage 16; 17: stage 17; 18: stage 18; 19: stage 19; 20: stage 20; 21: stage 21. Different letters and * indicate significant differences (P < 0.05). |
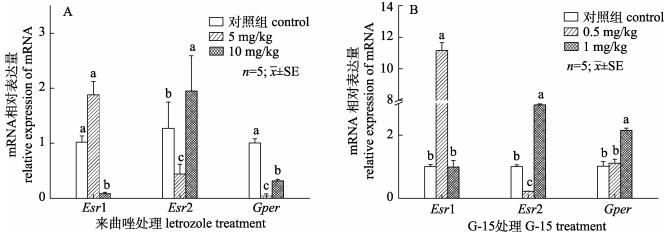
对雄鳖腹腔注射Letrozole和G-15后, 检测Esrs和Gper表达量变化情况。结果显示, Letrozole处理组中Gper表达量显著降低, 低浓度处理组中表达量最低; Esr1表达量在低浓度处理组中显著升高在高浓度处理组中显著下降; Esr2表达变化情况与Esr1基因相反(图 5A)。G-15处理组中, 高浓度处理组Gper表达量显著升高(P < 0.05); Esr1在高浓度处理组表达量显著升高, 低浓度处理组显著下降至对照组相同表达水平; Esr2在低浓度组显著下降, 高浓度组显著上升(图 5B)。
|
图 5 注射Letrozole及G-15后对中华鳖精巢中雌激素受体表达的影响 A.来曲唑处理后对雌激素受体的表达影响; B. G-15处理后对雌激素受体的表达影响; 不同字母代表显著性差异(P < 0.05). Fig.5 Effects of Letrozole or G-15 injection on estrogen receptor expression levels in testis of Pelodiscus sinensis A. The relative expression of estrogen receptor after injected Letrozole; B. The relative expression of estrogen receptor after injected G-15. Different letters indicate significant differences (P < 0.05). |
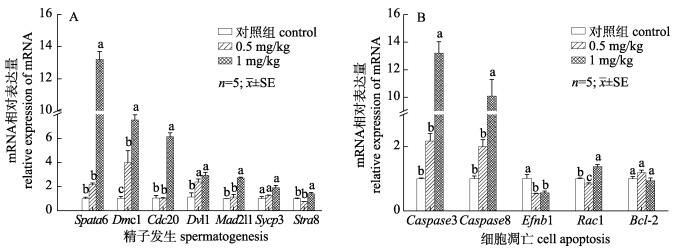
检测注射G-15后对精巢中精子发生及细胞凋亡相关基因表达的影响, 结果显示, G-15处理能够显著促进精巢中精子发生相关基因的表达: Spata6、Dmc1、Cdc20、Dvl1、Mad2l1、Stra8表达量显著升高(P < 0.05), Sycp3表达量有上升趋势(P > 0.05, 图 6A)。促凋亡细胞Caspase3、Caspase8表达量显著升高(P < 0.05)、抗凋亡基因Efnb1表达量明显降低、Rac1表达量在0.5 mg/kg组显著降低, 在1 mg/kg显著升高、Bcl-2没有明显变化(P > 0.05, 图 6B)。
|
图 6 注射G-15后对中华鳖精子发生及细胞凋亡基因表达的影响 A.精子发生基因表达量变化; B.细胞凋亡基因表达量变化; 不同字母代表显著性差异(P < 0.05). Fig.6 Effects of G-15 injection on spermatogenesis and cell apoptosis genes expression levels in testis of Pelodiscus sinensis A. The relative expression of spermatogenesis genes after injected G-15; B. The relative expression of cell apoptosis genes after injected G-15. Different letters indicate significant differences (P < 0.05). |
Gper属于Gpcr蛋白家族, 其具有相似的蛋白结构: 7个跨膜结构域以及A类亚家族特有的DRY三联体结构[20]。DRY结构在维持蛋白结构稳定、受体活化、配体结合以及信号传导中具有重要作用, 暗示了Gper功能的保守性[21]。本研究采用RACE技术从中华鳖脑中克隆了Gper cDNA序列全长, 其中开放阅读框长1077 bp, 编码1个由358个氨基酸组成的蛋白质, 其蛋白具有上述结构特征; 邻接法构建系统发育树发现其亲缘关系与龟类最近, 其次是鸟类和爬行类(图 3), 与中华鳖MyD88[22]、Tf [23]等基因研究结果相似, 符合物种的一般进化规律。以上结果表明克隆得到的cDNA序列为中华鳖Gper基因。
Gper作为雌激素受体在机体各组织中广泛表达。小鼠与斑马鱼中, Gper主要表达在脑与性腺中, 其不仅参与中枢神经的发育与修复, 还参与调控生殖细胞增殖和成熟[14, 24]。本研究中Gper的组织差异表达分析表明中华鳖Gper在脑中表达量最高, 其次为性腺, 卵巢的表达量是精巢的3倍(图 4A), 与其他物种中研究结果类似, 暗示了Gper在中华鳖脑及性腺发育的重要地位。分析Gper在性腺分化不同时期的表达模式发现, 27 ℃和32 ℃两个不同孵化条件下, Gper的表达模式相似:在性腺分化初期表达量最高, 性腺分化中后期表达量显著下降(图 4B)。芳香化酶是雄激素转化为雌激素的限速酶, 是性别分化的关键因素[25]。包海声等[26]研究发现中华鳖芳香化酶基因(Cyp19a1)在性腺分化阶段开始大量表达。原鸡中, Cyp19a1在性腺分化阶段表达量升高, 同时Gper表达量降低[16]。这一结果与中华鳖Cyp19a1、Gper性腺分化表达模式一致。在原鸡性腺分化前, Esrs不表达, Gper表达显著高于性别分化阶段, 雌激素通过Gper刺激原始生殖细胞增殖[11]。因此Gper可能通过原始生殖细胞增殖途径参与中华鳖早期性腺分化过程。此外, 32 ℃时Gper表达显著降低的时间早于27 ℃, 这可能由于在同一孵化时长下, 32 ℃孵化条件下中华鳖胚胎发育速度比27 ℃条件下快[27], 因此Gper表达量变化更快。
雌激素通过Esrs和Gper参与调控动物精巢发育, 本实验采用腹腔注射Letrozole和G-15研究Gper在精巢中的功能。Letrozole可抑制芳香化酶活性, 降低个体中雌激素水平[25]。Letrozole处理后, 中华鳖睾丸Gper表达量显著降低(P < 0.05), Esrs在不同剂量处理组中表现出相反的变化趋势。G-15处理后, Gper表达量上升, 可能是由于抑制Gper蛋白活性后机体产生应激反应[28]。Esrs不仅介导雌激素通过基因组途径发挥功能, 也可以介导非基因组通路的功能[29], 与Gper存在功能重叠[30]。体外与活体实验中, G-15抑制Gper活性会导致小鼠Esr1与Esr2表达增加[31]。本实验中, Esr1和Esr2表达量分别在0.5 mg/kg处理组和1 mg/kg处理组中显著上升(P < 0.05), 可能是由于机体通过提高Esrs的表达弥补Gper功能的缺失。
在此前的研究表明, 在睾丸体细胞及各级生精细胞都检测到Gper表达[15], 通过体外培养细胞系的方法发现Gper可能参与调控小鼠睾丸支持细胞、粗线期精母细胞及圆形精子的增殖与凋亡过程[32-34]。原鸡中研究结果显示, Gper可能通过Egfr/Akt/β-catenin信号通路调控原始生殖细胞的增殖与凋亡[11], 以上结果表明Gper可能通过参与细胞增殖与凋亡调控精子发生过程。本研究中通过检测G-15处理组中精子发生及细胞凋亡相关基因表达水平的变化研究Gper在雄鳖生殖过程中的作用。Spata6、Dmc1、Cdc20、Dvl1、Mad2l1、Stra8等是调控精子发生的关键基因[35-37], Caspase3和Caspase8参与调节精子发生过程中的细胞凋亡途径[38]。G-15处理后, 精子发生关键基因表达量明显升高; 同时, Caspase3、Caspase8表达量显著上升(P < 0.05, 图 6)。基因转录水平结果表明抑制Gper功能后, 对精子发生过程的关键基因及细胞凋亡基因的表达造成一定影响, 暗示了Gper可能参与雄鳖精子发生, 但其具体影响机制还需深入研究。
总之, 本研究克隆得到中华鳖Gper cDNA全长序列, 其编码的蛋白具有Gpcr家族典型的7个跨膜结构域和DRY三联体结构, 在进化上与龟鳖类最近。Gper在脑与性腺中表达量最高, 在性腺分化的关键时期表达量逐渐下降, 并且可能参与调控雄鳖精子发生与细胞凋亡过程, 为进一步探索Gper在中华鳖性别分化及精子发生中的功能奠定研究基础。
| [1] |
Szego C M, Davis J S. Adenosine 3', 5'-monophosphate in rat uterus:Acute elevation by estrogen[J]. Proceedings of the National Academy of Sciences of the United States of America, 1967, 58(4): 1711-1718. DOI:10.1073/pnas.58.4.1711 |
| [2] |
Pietras R J, Szego C M. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells[J]. Nature, 1977, 265(5589): 69-72. DOI:10.1038/265069a0 |
| [3] |
Vasudevan N, Pfaff D W. Membrane-initiated actions of estrogens in neuroendocrinology:Emerging principles[J]. Endocrine Reviews, 2007, 28(1): 1-19. |
| [4] |
O'Dowd B F, Nguyen T, Marchese A, et al. Discovery of three novel G-protein-coupled receptor genes[J]. Genomics, 1998, 47(2): 310-313. |
| [5] |
Zhang J D. Establishment and application of a detection model for G protein-coupled receptor signaling pathways and drug screening for orphan GPCRs[D]. Nanchang: Nanchang University, 2016. [张建东. GPCR信号通路研究模型的建立与应用及孤儿受体的药物筛选[D].南昌: 南昌大学, 2016.] http://d.wanfangdata.com.cn/thesis/D01017067
|
| [6] |
Rosenbaum D M, Rasmussen S G F, Kobilka B K. The structure and function of G-protein-coupled receptors[J]. Nature, 2009, 459(7245): 356-363. DOI:10.1038/nature08144 |
| [7] |
Kotula-Balak M, Pawlicki P, Milon A, et al. The role of G-protein-coupled membrane estrogen receptor in mouse Leydig cell function-in vivo and in vitro evaluation[J]. Cell and Tissue Research, 2018, 374(2): 389-412. |
| [8] |
Zhu D D, Yang L S, Huang J H, et al. The comprehensive expression analysis of the G protein-coupled receptor from Penaeus monodon indicating it participates in innate immunity and anti-ammonia nitrogen stress[J]. Fish & Shellfish Immunology, 2018, 75: 17-26. |
| [9] |
Prossnitz E R, Hathaway H J. What have we learned about GPER function in physiology and disease from knockout mice?[J]. The Journal of Steroid Biochemistry and Molecular Biology, 2015, 153: 114-126. DOI:10.1016/j.jsbmb.2015.06.014 |
| [10] |
Prossnitz E R, Barton M. Signaling, physiological functions and clinical relevance of the G protein-coupled estrogen receptor GPER[J]. Prostaglandins & Other Lipid Mediators, 2009, 89(3-4): 89-97. |
| [11] |
Ge C T, Yu M L, Zhang C Q. G protein-coupled receptor 30 mediates estrogen-induced proliferation of primordial germ cells via EGFR/Akt/β-catenin signaling pathway[J]. Endocrinology, 2012, 153(7): 3504-3516. DOI:10.1210/en.2012-1200 |
| [12] |
Wang C, Prossnitz E R, Roy S K. G protein-coupled receptor 30 expression is required for estrogen stimulation of primordial follicle formation in the hamster ovary[J]. Endocrinology, 2008, 149(9): 4452-4461. DOI:10.1210/en.2008-0441 |
| [13] |
Pang Y F, Dong J, Thomas P. Estrogen signaling characteristics of Atlantic croaker G protein-coupled receptor 30(GPR30) and evidence it is involved in maintenance of oocyte meiotic arrest[J]. Endocrinology, 2008, 149(7): 3410-3426. DOI:10.1210/en.2007-1663 |
| [14] |
Pang Y F, Thomas P. Involvement of estradiol-17β and its membrane receptor, G protein coupled receptor 30(GPR30) in regulation of oocyte maturation in zebrafish, Danio rario[J]. General and Comparative Endocrinology, 2009, 161(1): 58-61. |
| [15] |
Liu X C, Zhu P, Sham K W Y, et al. Identification of a membrane estrogen receptor in zebrafish with homology to mammalian GPER and its high expression in early germ cells of the testis[J]. Biology of Reproduction, 2009, 80(6): 1253-1261. |
| [16] |
Sirianni R, Chimento A, Ruggiero C, et al. The novel estrogen receptor, G protein-coupled receptor 30, mediates the proliferative effects induced by 17β-estradiol on mouse spermatogonial GC-1 cell line[J]. Endocrinology, 2008, 149(10): 5043-5051. DOI:10.1210/en.2007-1593 |
| [17] |
Morini M, Peñaranda D S, Vílchez M C, et al. The expression of nuclear and membrane estrogen receptors in the European eel throughout spermatogenesis[J]. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology, 2017, 203: 91-99. |
| [18] |
Long X W, Wu R F, Ma N, et al. Comparison of biological indices and nutritional composition of male Chinese soft-shelled turtle, Trionyx sinensis, reared in a greenhouse and eco-pond for Chinese mitten crab, Eriocheir sinensis[J]. Journal of Fishery Sciences of China, 2017, 24(1): 100-108. [龙晓文, 吴仁福, 麻楠, 等. 中华绒螯蟹池塘套养与温室养殖的中华鳖雄体生物学指数和营养成分比较[J]. 中国水产科学, 2017, 24(1): 100-108.] |
| [19] |
Tokita M, Kuratani S. Normal embryonic stages of the Chinese softshelled turtle Pelodiscus sinensis (Trionychidae)[J]. Zoological Science, 2001, 18(5): 705-715. DOI:10.2108/zsj.18.705 |
| [20] |
Erlandson S C, McMahon C, Kruse A C. Structural basis for G protein-coupled receptor signaling[J]. Annual Review of Biophysics, 2018, 47: 1-18. DOI:10.1146/annurev-biophys-070317-032931 |
| [21] |
Strader C D, Fong T M, Tota M R, et al. Structure and function of G protein-coupled receptors[J]. Annual Review of Biochemistry, 1994, 63(1): 101-132. DOI:10.1146/annurev.bi.63.070194.000533 |
| [22] |
Zhu B L, Li J, Fang W H, et al. Cloning of MyD88 partial cDNA sequence of Chinese soft-shelled turtle (Trionyx sinensis) and its expression in different tissues[J]. Journal of Fisheries of China, 2010, 34(7): 1018-1024. [朱炳林, 李俊, 方维焕, 等. 中华鳖MyD88部分序列克隆及其在组织中的表达差异分析[J]. 水产学报, 2010, 34(7): 1018-1024.] |
| [23] |
Li W, Shi Y, Zhao J, et al. Molecular characteristics and the expression of transferrin gene in Chinese soft-shell turtle[J]. Acta Hydrobiologica Sinica, 2015, 39(3): 482-489. [李伟, 史燕, 赵建, 等. 中华鳖转铁蛋白基因序列特征及表达研究[J]. 水生生物学报, 2015, 39(3): 482-489.] |
| [24] |
Fan Y Q, Liang C L, Xiong Y X, et al. Role of GPER- mediated estrogens in alleviating nerve injury in ischemic stroke rats and its mechanism[J]. Chinese Journal of Geriatric Heart Brain and Vessel Diseases, 2016, 18(10): 1086-1089. [范雅倩, 梁楚玲, 熊逸潇, 等. G蛋白偶联雌激素受体介导的雌激素减轻缺血性脑卒中大鼠神经损伤的作用及机制探讨[J]. 中华老年心脑血管病杂志, 2016, 18(10): 1086-1089. DOI:10.3969/j.issn.1009-0126.2016.10.021] |
| [25] |
Li Y J, Wu L M, Wang L, et al. Molecular cloning and characterization of Cyp19a1b gene and the effect of Letrozole on its expression in Carassius auratus[J]. Journal of Fisheries of China, 2018, 42(8): 1169-1180. [李永婧, 吴利敏, 王磊, 等. 淇河鲫Cyp19a1b基因的克隆表达及芳香化酶抑制剂对其表达的影响[J]. 水产学报, 2018, 42(8): 1169-1180.] |
| [26] |
Bao H S, Cai H, Han W, et al. Functional characterization of Cyp19a1in female sexual differentiation in Pelodiscus sinensis[J]. Scientia Sinica (Vitae), 2017, 47(6): 640-649. [包海声, 蔡晗, 韩伟, 等. Cyp19a1基因在中华鳖早期卵巢分化中的功能研究[J]. 中国科学:生命科学, 2017, 47(6): 60-649.] |
| [27] |
Zhu A L, Shi Y, Zhu X P, et al. Effects of different temperature on embryonic development, hatchling traits and hatchling moving of Trionyx sinensis[J]. Genomics and Applied Biology, 2013, 32(3): 303-307. [朱阿莉, 史燕, 朱新平, 等. 温度对中华鳖胚胎发育和初生幼体形态特征及活动能力的影响[J]. 基因组学与应用生物学, 2013, 32(3): 303-307.] |
| [28] |
Méndez-Luna D, Martínez-Archundia M, Maroun R C, et al. Deciphering the GPER/GPR30-agonist and antagonists interactions using molecular modeling studies, molecular dynamics, and docking simulations[J]. Journal of Biomolecular Structure and Dynamics, 2015, 33(10): 2161-2172. DOI:10.1080/07391102.2014.994102 |
| [29] |
Dostalova P, Zatecka E, Dvorakova-Hortova K. Of oestrogens and sperm:A review of the roles of oestrogens and oestrogen receptors in male reproduction[J]. International Journal of Molecular Sciences, 2017, 18(5): 904. DOI:10.3390/ijms18050904 |
| [30] |
Fietz D, Ratzenböck C, Hartmann K, et al. Expression pattern of estrogen receptors α and β and G-protein-coupled estrogen receptor 1 in the human testis[J]. Histochemistry and Cell Biology, 2014, 142(4): 421-432. |
| [31] |
Kotula-Balak M, Milon A, Pawlicki P, et al. Insights into the role of estrogen-related receptors α, β and γ in tumor Leydig cells[J]. Tissue and Cell, 2018, 52: 78-91. DOI:10.1016/j.tice.2018.04.003 |
| [32] |
Royer C, Lucas T F G, Lazari M F M, et al. 17 beta-estradiol signaling and regulation of proliferation and apoptosis of rat Sertoli cells[J]. Biology of Reproduction, 2012, 86(4): 108. |
| [33] |
Chimento A, Sirianni R, Delalande C, et al. 17β-estradiol activates rapid signaling pathways involved in rat pachytene spermatocytes apoptosis through GPR30 and ERα[J]. Molecular and Cellular Endocrinology, 2010, 320(1-2): 136-144. DOI:10.1016/j.mce.2010.01.035 |
| [34] |
Chimento A, Sirianni R, Zolea F, et al. Gper and ESRs are expressed in rat round spermatids and mediate oestrogen-dependent rapid pathways modulating expression of cyclin B1 and Bax[J]. International Journal of Andrology, 2011, 34(5pt1): 420-429. DOI:10.1111/j.1365-2605.2010.01100.x |
| [35] |
Anderson E L, Baltus A E, Roepers-Gajadien H L, et al. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice[J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(39): 14976-14980. DOI:10.1073/pnas.0807297105 |
| [36] |
Jin F, Hamada M, Malureanu L, et al. Cdc20 is critical for meiosis Ⅰ and fertility of female mice[J]. PLoS Genetics, 2010, 6(9): e1001147. DOI:10.1371/journal.pgen.1001147 |
| [37] |
Yuan S Q, Stratton C J, Bao J Q, et al. Spata6 is required for normal assembly of the sperm connecting piece and tight head-tail conjunction[J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(5): E430-E439. DOI:10.1073/pnas.1424648112 |
| [38] |
Royère D, Guerif F, Rochereau de Reviers M T, et al. Apoptosis in the male gonad[J]. Contraception Fertilité Sexualité, 1998, 26(7-8): 517-521. |
 2020, Vol. 27
2020, Vol. 27

