2. 中国水产科学研究院淡水渔业研究中心, 农业农村部淡水渔业与种质资源利用重点实验室, 江苏 无锡 214081
2. Freshwater Fisheries Research Center, Chinese Academg of Fishery Sciences; Key Laboratory of Freshwater Fisheries and Germplasm Resources Utilization, Ministry of Agriculture and Rural Affairs, Wuxi 214081, China
溶解氧(dissolved oxygen, DO)对水生动物而言至关重要, 是其获得氧气的主要来源以及赖以生存的前提条件, 在其生长、发育和能量代谢等方面发挥重要作用。近年来, 养殖池塘中低氧现象日益频繁, 水产品在长途运输过程中也常面临缺氧难题[1]。当低氧胁迫时间过长或者胁迫压力过大时, 会造成水生动物机体不可逆损伤使其无法维持正常的能量代谢平衡, 最终导致免疫力下降、代谢紊乱等, 甚至导致个体死亡[2]。迄今为止, 有关甲壳动物低氧胁迫响应的研究已从行为反应、生长繁殖和免疫机能等生化层面[3-8], 延伸至低氧信号传导、基因转录调控和关键蛋白功能等分子层面[9-12], 但低氧胁迫对经济虾蟹组织结构的影响鲜有报道, 仅见魏琳等[13]比较研究了低氧胁迫对2个凡纳滨对虾品系的肌肉和鳃组织线粒体超微结构的影响。
日本沼虾(Macrobrachium nipponense)俗称河虾、青虾, 隶属于十足目(Decapoda), 长臂虾科(Palaemonidae), 沼虾属, 据渔业年鉴统计, 2016年全国日本沼虾养殖总产量已达到27.26万t[14], 是中国产量较高的淡水养殖经济虾类之一。近年来由于日本沼虾养殖规模的扩大、集约化程度的提高导致池塘养殖密度过大, 加之日本沼虾自身不耐低氧的生物学特性, 水体的溶解氧含量已成为影响日本沼虾产量的重要环境因素之一[15]。近年来, 本实验室对日本沼虾在低氧应激的响应及其耐受机制方面进行了大量研究, 但主要集中在基因克隆与表达[16-18]、代谢组学相关分析[19]等方面, 有关低氧对日本沼虾组织结构影响的研究较少, 仅见慢性低氧胁迫对日本沼虾肝胰腺和鳃组织结构的影响[20], 尚未见急性低氧胁迫对日本沼虾组织结构变化影响的研究。
本研究首先通过实验确定了低氧胁迫下日本沼虾8 h半致死浓度, 依据半致死浓度设置胁迫浓度, 分析低氧及复氧条件下日本沼虾抗氧化酶活力的变化, 同时结合肌肉、肝胰腺和鳃的组织结构变化, 从生理和组织水平深入分析日本沼虾低氧应激的生理响应过程, 为日本沼虾健康养殖以及耐低氧群体选育提供科学依据。
1 材料与方法 1.1 实验用虾本实验用虾为人工养殖日本沼虾, 取自靠近无锡太湖湖区的大浦养殖基地, 选取体重(2.0±0.2) g健康活泼的日本沼虾在室内玻璃缸(120 L, 93.5 cm×43.5 cm×30.5 cm)中暂养14 d, 按其体重的3%~5%定时投喂配合饲料, 每2~3 d换水1次, 换水量为总水体体积50%。
1.2 实验方法 1.2.1 8 h半致死实验通过预实验测定日本沼虾水体溶氧耐受范围, 并根据实验所需设置实验水体溶氧梯度, 实验过程中每隔2~3 h用Mettler Seven2Go Pro S9型便携式溶氧仪(Mettler公司, 瑞士)监测水缸中DO浓度。通过补充氮气以调节水中的DO质量浓度, 使其维持在设定范围内。确定日本沼虾8 h全部死亡和8 h全部存活的溶氧量在0.4~6 mg/L。在此基础上设置0.5 mg/L、1 mg/L、2 mg/L、6 mg/L共4个溶氧梯度, 每个梯度设置3个平行组, 每个平行放10尾虾。依照文献[21]随时观察并记录日本沼虾活动情况及死亡数量, 调整水体溶氧含量并及时捞出死虾, 采用寇氏法计算日本沼虾低氧胁迫8 h半致死浓度。
1.2.2 样品的采集与处理根据急性低氧胁迫下日本沼虾8 h半致死浓度, 设置对照组[DO (6.0± 0.2) mg/L]及实验组[DO (2.0±0.2) mg/L]每组各设3个平行组, 每个平行放养35尾虾, 每隔2~3 h测定并调整DO值。水温为(23±0.10)℃, pH 8.01± 0.03。分别在低氧0 h、6 h、12 h、24 h及复氧1 h、6 h、12 h、24 h时, 从各缸随机选取5尾日本沼虾取样, 获取完整的鳃、肌肉和肝胰腺组织用液氮速冻后, 放入–80℃冰箱中保存用于酶活力的测定, 剩余肌肉、鳃和肝胰腺组织放入Bouin’s液中固定用于组织切片的制作与观察。
1.2.3 酶活力测定鳃、肌肉及肝胰腺样品解冻后, 准确称取组织重量, 按重量体积比1 ː 9加入生理盐水将其制成10%的组织匀浆, 离心并取上清液待测。其中虾肌肉再用生理盐水稀释20倍, 取样50 μL测定, 日本沼虾肝胰腺再用生理盐水稀释10倍, 取样50 μL测定。
1.2.4 鳃、肌肉和肝胰腺组织的石蜡切片制作固定好的样品首先经过酒精梯度脱水, 二甲苯透明, 石蜡包埋, 轮转式切片机连续切片, 切片约5 μm厚度, 用苏木精–伊红进行染色, 中性树胶封片, OlympusBX-50型荧光显微镜观察并拍照。
1.3 数据分析采用SPSS Statistics 22.0分析数据, 实验结果以平均数±标准误(x±SE)来表示。同一个时间点两组间的数据运用独立样本t检验进行比较, 同一组不同时间点的数据运用One-Way ANOVA进行比较且差异显著时采用Duncan’s多重比较, P < 0.05认为差异显著。
2 结果与分析: 2.1 日本沼虾8 h半致死浓度(LC50)的测定如表 1所示, 日本沼虾低氧胁迫8 h的死亡率随溶解氧浓度的降低逐渐上升(表 1), 以此数据为基础, 通过运用寇氏法公式进行计算求得日本沼虾8 h水体溶解氧半致死浓度为0.85 mg/L。
|
|
表 1 不同溶氧水平对日本沼虾死亡率的影响 Tab.1 Effect of different DO level on the mortality of Macrobrachium nipponense |
由图 1可知, 随着低氧胁迫时间的延长, 实验组日本沼虾肌肉SOD、CAT、GPX 3种酶活力均呈现先升高后降低的变化趋势, 且在低氧胁迫12 h (H12)时均显著升高(P < 0.05)并达到最大值; 低氧胁迫24 h (H24)时3种抗氧化酶活力均有下降, 其中实验组SOD酶活力显著高于对照组(P < 0.05, 图 1A), CAT、GPX酶活力显著低于对照组(P < 0.05, 图 1B, 图 1C); 复氧期间, 实验组SOD酶活力与CAT酶活力变化趋势相反, 复氧24 h (F24)时实验组SOD、CAT酶活力(图 1A, 图 1B)均与对照组无显著差异(P > 0.05), 实验组GPX酶活力呈现先升高后降低再升高的变化规律, 复氧24 h时实验组GPX酶活力显著低于对照组(P < 0.05)。
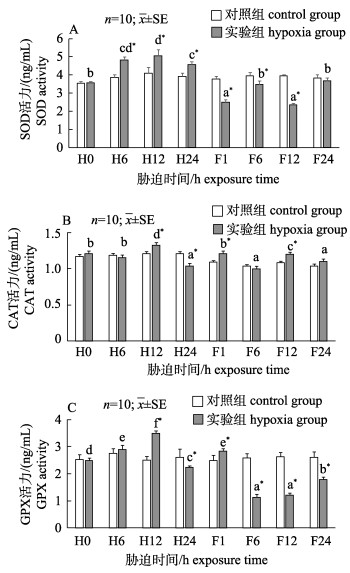
|
图 1 低氧及其恢复对日本沼虾肌肉组织SOD (A), CAT (B)和GPX (C)活力的影响
不同小写字母表示同一处理不同时间点之间差异显著(P < 0.05), *表示实验组和对照组在胁迫后同一时间点差异显著(P < 0.05). H0, H6, H12, H24分别代表低氧胁迫0 h, 6 h, 12 h, 24 h; F1, F6, F12, F24分别代表溶氧恢复1 h, 6 h, 12 h, 24 h. Fig.1 Effect of hypoxia and reoxygenation on SOD(A), CAT(B) and GPX (C) activities in muscle of Macrobrachium nipponense Different lowercase superscripts mean significant differences (P < 0.05) within the same treatment among different times. *means significant differences (P < 0.05) between hypoxia group and the control group at the same time. H0, H6, H12, H24 represent hypoxia stress at 0 h, 6 h, 12 h, 24 h, respectively; F1, F6, F12, F24 represent reoxygenation at 1 h, 6 h, 12 h, 24 h, respectively. |
由图 2可知, 随着低氧胁迫时间的延长, 实验组日本沼虾鳃组织SOD、CAT酶活力呈现出先升高后下降趋势, 且在低氧胁迫12 h (H12)时均显著升高(P < 0.05)并达到最大值, 低氧胁迫24 h (H24)时SOD、CAT 2种抗氧化酶活力均有下降, 其中SOD酶活力显著高于对照组(P < 0.05, 图 2A), CAT酶活力显著低于对照组(P < 0.05, 图 2B); 实验组GPX酶活力在低氧胁迫期间逐渐升高, 在低氧处理24 h (H24)时实验组GPX酶活力达到最大值并显著高于对照组(P < 0.05, 图 2C)。复氧1 h (F1)时SOD酶活力显著高于对照组, 随后实验组SOD酶活力与对照组无显著差异(P > 0.05);实验组CAT酶活力在复氧期间整体呈现出下降的趋势, 复氧1 h (F1) CAT酶活力最高且显著高于对照组(P < 0.05), 复氧24 h时实验组与对照组无显著差异(P > 0.05); GPX酶活力在复氧期间逐渐升高且复氧24 h时(F24)显著高于对照组(P < 0.05)。
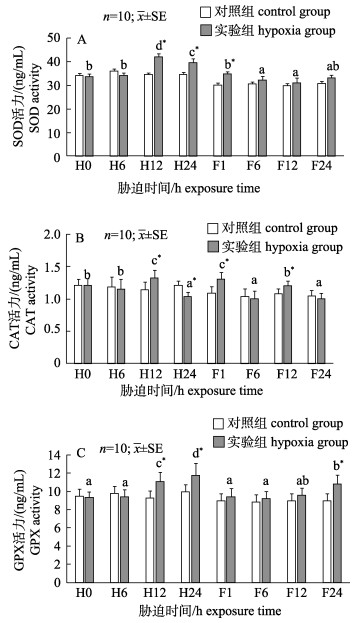
|
图 2 低氧及其恢复对日本沼虾鳃组织SOD (A), CAT (B)和GPX (C)活力的影响
不同小写字母表示同一处理不同时间点之间差异显著(P < 0.05),
*表示实验组和对照组在胁迫后同一时间点差异显著(P < 0.05). H0, H6, H12, H24分别代表低氧胁迫0 h, 6 h, 12 h, 24 h; F1, F6, F12, F24分别代表溶氧恢复1 h, 6 h, 12 h, 24 h. Fig.2 Effect of hypoxia and reoxygenation on SOD (A), CAT (B) and GPX (C) activities in gill of Macrobrachium nipponense Different lowercase superscripts mean significant differences (P < 0.05) within the same treatment among different times. *means significant differences (P < 0.05) between hypoxia group and the control group at the same time. H0, H6, H12, H24 represent hypoxia stress at 0 h, 6 h, 12 h, 24 h, respectively; F1, F6, F12, F24 represent reoxygenation at 1 h, 6 h, 12 h, 24 h, respectively. |
由图 3可知, 实验组肝胰腺SOD酶活力随着胁迫时间的延长呈先升高后降低趋势且显著高于对照组(P < 0.05, 图 3A), 复氧期间实验组SOD酶活力逐渐下降且复氧24 h时显著低于对照组(P < 0.05)。实验组肝胰腺组织中CAT酶活力在低氧胁迫期间先升高后降低, 低氧6 h时实验组酶活力达到最大值并显著高于对照组(P < 0.05, 图 3B), 低氧胁迫12 h时CAT酶活力显著低于对照组(P < 0.05), 低氧胁迫24 h实验组CAT酶活力有所上升且与对照组无显著差异(P > 0.05);复氧期间实验组CAT酶活力呈波动性变化, 复氧1 h和12 h时实验组CAT酶活力与对照组无显著差异(P > 0.05), 复氧6 h和24 h时实验组CAT酶活力显著高于对照组(P < 0.05)。实验组肝胰腺组织中GPX酶活力在低氧胁迫期间先升高后降低(图 3C), 低氧6 h实验组GPX酶活力达到最大值后显著下降(P < 0.05), 复氧期间实验组GPX酶活力逐渐下降且复氧24 h显著低于对照组(P < 0.05)。实验组肝胰腺组织中MDA含量在低氧及复氧期间均表现出先升高后下降的趋势并显著高于对照组(P < 0.05, 图 3D)。
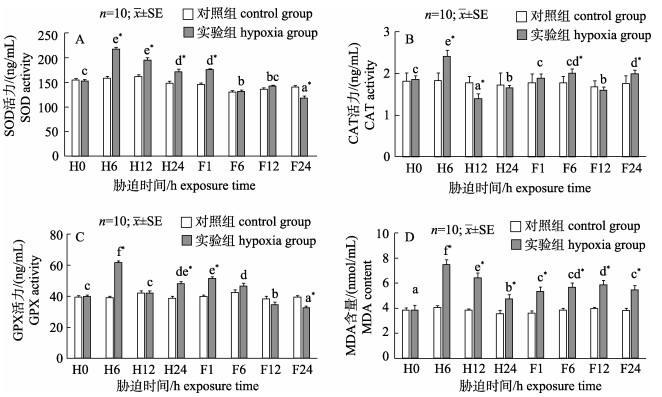
|
图 3 低氧及其恢复对日本沼虾肝胰腺SOD(A), CAT(B), GPX (C)活力及MDA(D)含量的影响
不同小写字母表示同一处理不同时间点之间差异显著(P < 0.05);
*表示实验组和对照组在胁迫后同一时间点差异显著(P < 0.05). H0, H6, H12, H24分别代表低氧胁迫0 h, 6 h, 12 h, 24 h; F1, F6, F12, F24分别代表溶氧恢复1 h, 6 h, 12 h, 24 h. Fig.3 Effect of hypoxia and reoxygenation on SOD (A), CAT (B), GPX (C) activities and MDA (D) content in hepatopancreas of Macrobrachium nipponense Different lowercase superscripts mean significant differences (P < 0.05) within the same treatment among different times. *means significant differences (P < 0.05) between hypoxia group and the control group at the same time. H0, H6, H12, H24 represent hypoxia stress at 0 h, 6 h, 12 h, 24 h, respectively; F1, F6, F12, F24 represent reoxygenation at 1 h, 6 h, 12 h, 24 h, respectively. |
如图 4所示, 日本沼虾肌肉组织显微结构在经过低氧胁迫及恢复时, 其肌肉组织结构排列依然较为整齐, 肌纤维数目均无明显差异, 肌纤维间隙未出现显著变化。
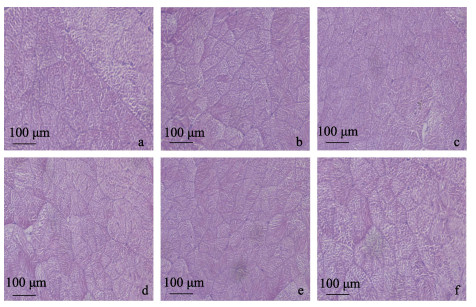
|
图 4 低氧胁迫及复氧对日本沼虾肌肉显微结构的影响 a.低氧胁迫0 h (对照组)肌肉组织显微结构图, HE染色; b.低氧胁迫12 h肌肉组织显微结构图, HE染色; c.低氧胁迫24 h肌肉组织显微结构图, HE染色; d.复氧1 h后肌肉组织显微结构图, HE染色; e.复氧12 h后肌肉组织显微结构图, HE染色; f.复氧24 h后肌肉组织显微结构图, HE染色. Fig.4 Effect of hypoxia and reoxygenation on muscle microstructure of Macrobrachium nipponense a. The microscopical muscle structure of Macrobrachium nipponense in response to hypoxia at 0 h, HE; b. The microscopical muscle structure of Macrobrachium nipponense in response to hypoxia at 12 h; HE; c. The microscopical muscle structure of Macrobrachium nipponense in response to hypoxia at 24 h, HE; d. The microscopical muscle structure of Macrobrachium nipponense in response to reoxygenation at 1 h, HE; e. The microscopical muscle structure of Macrobrachium nipponense in response to reoxygenation at 12 h, HE; f. The microscopical muscle structure of Macrobrachium nipponense in response to reoxygenation at 24 h, HE. |
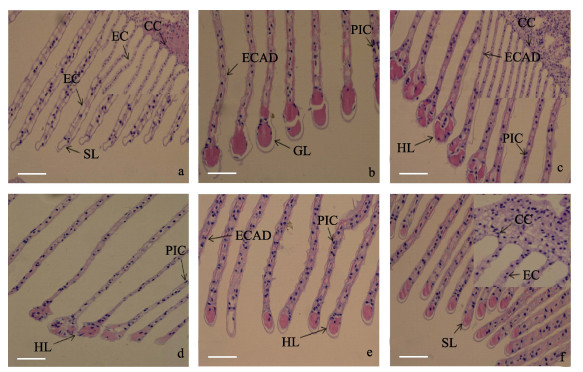
胁迫0 h(图 5a)时, 日本沼虾鳃组织结构完整, 呼吸上皮细胞及支柱细胞排列整齐, 细胞结构以及次级层片形态结构正常。在低氧胁迫12 h (图 5b)时上皮与支柱细胞排列发生紊乱现象, 次级层片开始肥大。低氧胁迫24 h (图 5c)时泌氯细胞数目增加且形态由椭圆形向圆形转化, 以此增加细胞接触面积, 次级层片肥大加剧, 且有红细胞流入。复氧1 h (图 5d)时次级层片肥大状态有所恢复, 且次级层片内部血细胞数量有所减少。复氧12 h (图 5e)时次级层片开始萎缩, 复氧24 h (图 5f)后泌氯细胞形态逐渐向椭圆形态恢复且数目逐渐减少, 上皮及支柱细胞排列逐渐恢复正常。
|
图 5 低氧胁迫及复氧对日本沼虾鳃丝显微结构的影响
a.低氧胁迫0 h (对照组)鳃丝组织显微结构图, HE染色; b.低氧胁迫12 h鳃丝组织显微结构图, HE染色; c.低氧胁迫24 h鳃丝组织显微结构图, HE染色; d.复氧1 h后鳃丝组织显微结构图, HE染色; e.复氧12 h后鳃丝组织显微结构图, HE染色; f.复氧24 h后鳃丝组织显微结构图, HE染色. CC:泌氯细胞; CL:次级片层卷曲; EC:上皮细胞; ECAD:上皮细胞排列紊乱; PIC:支柱细胞排列紊乱; SL:次级层片. Fig.5 Effect of hypoxia and reoxygenation on gill microstructure in Macrobrachium nipponense a. The microscopical gill structure of Macrobrachium nipponense in response to hypoxia at 0 h; b. The microscopical gill structure of Macrobrachium nipponense in response to hypoxia at 12 h, HE; c. The microscopical gill structure of Macrobrachium nipponense in response to hypoxia at 24 h, HE; d. The microscopical gill structure of Macrobrachium nipponense in response to reoxygenation at 1 h, HE; e. The microscopical gill structure of Macrobrachium nipponense in response to reoxygenation at 12 h, HE; f. The microscopical gill structure of Macrobrachium nipponense in response to reoxygenation at 24 h, HE. CC: chloride cells; CL: curling of secondary lamellae; EC: epithelial cells; ECAD: epithelial cells arranged disorder; PIC: pillar cells arranged disorder; SL: secondary lamellae. |
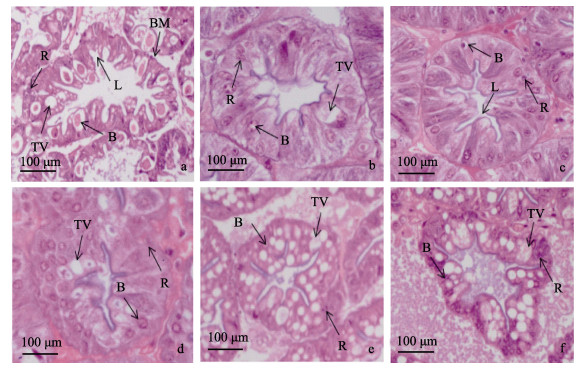
胁迫0 h(图 6a)时, 日本沼虾肝胰腺中细胞结构正常且分布较为均匀, 低氧胁迫12 h (图 6b)时B细胞及其内部运转泡体积明显减少, 运转泡内部颗粒物质逐渐消失, 低氧胁迫24 h (图 6c)时, R细胞数量相比对照组无显著变化, B细胞内部运转泡体积显著降低, 细胞数量相比对照组显著减少, 肝小管的形态结构明显收缩变形。复氧1 h (图 6d)时, B细胞数量及内部运转泡体积有所增加, 复氧12 h(图 6e)时, B细胞数量开始大量增加但其内部运转泡体积变化较小, 肝小管形态结构有所恢复, 复氧24 h (图 6f)时, R细胞数量与对照组相比并未出现明显减少, B细胞数量有所降低, 其内部运转泡示体积显著增加, 且其内部出现颗粒物质。
|
图 6 低氧胁迫及复氧对日本沼虾肝胰腺显微结构的影响
a.表示低氧胁迫0 h (对照组)肝胰腺组织显微结构图, HE染色; b.表示低氧胁迫12 h肝胰腺组织显微结构图, HE染色; c.表示低氧胁迫24 h肝胰腺组织显微结构图, HE染色; d.表示复氧1 h后肝胰腺组织显微结构图; e.表示复氧12 h后肝胰腺组织显微结构图, HE染色; f.表示复氧24 h后肝胰腺组织显微结构图. HE染色. B:分泌细胞, BM:基膜, L管腔, R:储存细胞, TV:转运泡. Fig.6 Effect of hypoxia and reoxygenation on hepatopancreas microstructure in Macrobrachium nipponense a. The microscopical hepatopancreas structure of Macrobrachium nipponense in response to hypoxia at 0 h, HE; b. The microscopical hepatopancreas structure of Macrobrachium nipponense in response to hypoxia at 12 h, HE; c. The microscopical hepatopancreas structure of Macrobrachium nipponense in response to hypoxia at 24 h, HE; d. The microscopical hepatopancreas structure of Macrobrachium nipponense in response to reoxygenation at 1 h, HE m; e. The microscopical hepatopancreas structure of Macrobrachium nipponense e in reoxygenation at 12 h, HE; f. The microscopical hepatopancreas structure of Macrobrachium nipponense in response to reoxygenation at 24 h, HE. B: secretory cells; BM: basement membrane; L: lumen; R: storage cells; TV: transferred vacuoles. |
水体溶氧过低会对水生生物个体生长发育造成直接或间接影响, 甚至将导致其生物个体死亡, 而水生生物的窒息点则能直观地反映出对低氧胁迫的耐受性[22]。本实验中日本沼虾的低氧胁迫8 h半致死浓度(LC50)为0.85 mg/L, 而据报道刀额对虾低氧胁迫8 h的LC50为0.77 mg/L[21]。日本沼虾低氧胁迫半致死浓度高于刀额对虾恰恰说明日本沼虾是低氧敏感虾类, 同时本研究发现低氧导致死亡主要发生在前5 h内, 当低氧胁迫达到8 h时, 其致死数基本不会发生改变, 这表明日本沼虾群体中存在耐低氧能力较强的个体, 它能够通过生理生化、呼吸代谢调节来逐渐适应低氧环境。
3.2 低氧胁迫对日本沼虾抗氧化酶活力的影响当机体受到胁迫时其体内ROS含量会迅速增加, 而未能被抗氧化系统及时清除的ROS将会促进细胞膜脂质过氧化物形成, 从而对机体造成氧化损伤[23]。具有清除氧自由基作用的抗氧化酶主要包括SOD、CAT和GPX, 酶系统可将超氧自由基(O2–)转化为对生物体无毒的H2O和O2[24-25]。因此, 低氧胁迫下日本沼虾体内SOD、CAT、GPX 3种抗氧化酶活力均显著升高, 表明由于低氧胁迫的影响造成日本沼虾体内ROS含量显著升高, 进而导致其相应抗氧化酶的活力增强, 这与南美白对虾(Penaeus vannamei)的研究结果相吻合[26]。虽然在胁迫前期抗氧化系统被激活, 但是MDA含量依然在显著提升, 说明机体仍然处于脂质过氧化状态。与对照组相比, 当低氧6 h时肝胰腺中CAT酶活性显著升高, 但当低氧12~ 24 h时肝胰腺中CAT酶活力显著下降, 其原因是SOD酶通过歧化反应产生过量的H2O2超出了其承受范围, 导致其活力受到抑制, 这与大黄鱼(Larimichthys crocea)在低氧胁迫后期结果相类似[27], 这也间接证实低氧会导致肝胰腺组织损伤。在日本沼虾复氧期间, 日本沼虾肌肉组织中SOD、GPX两种抗氧化酶活力显著降低, 这可能是由于复氧导致代谢速率忽然升高, 并抑制抗氧化系统发挥作用。在整个研究过程中, 鳃作为日本沼虾的呼吸器官, 其抗氧化酶活力变化范围小于肌肉和肝胰腺组织, 这可能得益于鳃组织在面对低氧环境时可以通过组织结构变化来获得更强的摄氧能力, 这与凡纳滨对虾和卵形鲳鲹的相关研究结果一致[28-30]。肝胰腺抗氧化酶活力远远高于肌肉和鳃组织, 这可能与肝胰腺在甲壳动物中具有消化、吸收、解毒等多方面作用且具有较高的代谢速率[31]相关。研究结果表明, 低氧胁迫前期体内抗氧化功能被激活, 胁迫后期肌肉与鳃组织抗氧化功能有所恢复, 低氧胁迫下抗氧化酶活力变化受生理功能及代谢速率的影响, 且存在一定的组织特异性。
3.3 低氧胁迫对肌肉、鳃和肝胰腺组织结构的影响在生物体面临低氧胁迫时, 其体内不同组织由于其结构与功能的不同而产生的应激反应也是不同的。本研究中, 肌肉组织结构在低氧胁迫与恢复过程中并未发现明显的组织结构上的变化。这一结果与团头鲂在面临低氧胁迫时的情况相类似[32]。鳃作为气体交换和离子调节的关键部位, 在水体生物受到环境胁迫后往往最先受到影响并造成损伤[33]。本研究中, 随着低氧胁迫时间的延长, 日本沼虾鳃组织结构形态发生改变, 包括其微血腔增大, 上皮与支柱细胞排列发生紊乱, 黏液分泌增加, 泌氯细胞数目增加, 次级层片肥大且有红细胞流入等现象, 而在复氧阶段其组织形态结构显著恢复, 这一结果与低氧胁迫下虹鳟(Oncorhynchus mykiss)[34]、鲫(Carassius auratus)[35]、青海湖裸鲤(Gymnocypris przewalskii)[36]等鳃组织结构变化规律的结果相一致。结合抗氧化酶活力变化可发现低氧胁迫对肌肉组织与鳃组织均产生了氧化应激反应, 但是肌肉组织并不能通过改变形态结构来减少其所面临的氧化压力, 而鳃组织则可以。日本沼虾肝胰腺主要细胞中分泌细胞(B细胞)与储存细胞(R细胞)数量较多[37]。在本研究中, 低氧胁迫导致日本沼虾肝胰腺内B细胞运转泡体积减小, 数量降低; 复氧情况下B细胞数量及运转泡体积逐渐恢复。而在克氏原螯虾[31]中低pH胁迫导致其肝胰腺B细胞数量增加且其细胞内运转泡体积增大; 三疣梭子蟹[38]在低盐暴露下R细胞减少, B细胞数量增加, 胞内运转泡体积增大; 中华绒螯蟹[39]在氨氮胁迫下B细胞及其运转泡数量和体积增加, 这些现象均与本研究中复氧阶段结果相类似。在复氧阶段, 相对低氧胁迫阶段B细胞数量及运转泡体积的均显著增加, 原因可能是随着氧气供应的恢复, 其分泌活动也逐渐恢复, 进而使其数量及体积逐渐增加。整个低氧–复氧阶段, R细胞数量均未发生明显变化, 表明急性低氧胁迫并不会对日本沼虾的营养物质储存产生显著影响。本研究可以看出低氧胁迫会对日本沼虾肌肉、鳃和肝胰腺等组织正常的生理代谢产生影响, 并对鳃及肝胰腺形态结构造成损伤, 当胁迫超出其组织承受范围时可能导致其代谢功能发生紊乱并可能导致个体的死亡。但是其影响可随着溶氧的恢复而逐渐得到恢复。肝胰腺组织在胁迫后期受到一定抑制,且24 h恢复期不足以让日本沼虾在低氧胁迫中完全恢复。
| [1] |
Costantini D, Rowe M, Butler M W, et al. From molecules to living systems:historical and contemporary issues in oxidative stress and antioxidant ecology[J]. Functional Ecology, 2010, 24(5): 950-959. DOI:10.1111/j.1365-2435.2010.01746.x |
| [2] |
Nordlie F G. Influences of body mass, temperature, oxygen tension, and salinity on respiratory oxygen consumption of cyprinodontoid fishes of three families[J]. Reviews in Fish Biology and Fisheries, 2014, 24(1): 269-315. DOI:10.1007/s11160-013-9331-9 |
| [3] |
Li Y H, Wei L, Cao J R, et al. Oxidative stress, DNA damage and antioxidant enzyme activities in the Pacific white shrimp (Litopenaeus vannamei) when exposed to hypoxia and reoxygenation[J]. Chemosphere, 2016, 144: 234-240. DOI:10.1016/j.chemosphere.2015.08.051 |
| [4] |
Parrilla-Taylor D P, Zenteno-Savín T. Antioxidant enzyme activities in Pacific white shrimp (Litopenaeus vannamei) in response to environmental hypoxia and reoxygenation[J]. Aquaculture, 2011, 318(3-4): 379-383. DOI:10.1016/j.aquaculture.2011.05.015 |
| [5] |
Qiu R J, Cheng Y X, Huang X X, et al. Effect of hypoxia on immunological, physiological response, and hepatopancreatic metabolism of juvenile Chinese mitten crab Eriocheir sinensis[J]. Aquaculture International, 2011, 19(2): 283-299. DOI:10.1007/s10499-010-9390-z |
| [6] |
Brown-Peterson N J, Manning C S, Patel V, et al. Effects of cyclic hypoxia on gene expression and reproduction in a grass shrimp, Palaemonetes pugio[J]. Biology Bulletin, 2008, 214(1): 6-16. |
| [7] |
Cheng W, Liu C H, Hsu J P, et al. Effect of hypoxia on the immune response of giant freshwater prawn Macrobrachium rosenbergii and its susceptibility to pathogen Enterococcus[J]. Fish & Shellfish Immunology, 2002, 13(5): 351-365. |
| [8] |
Li Z J. Effects of hypoxic stress on energy metabolism, respiratory metabolism and antioxidant metabolism of Eriocheir sinensis[D]. Baoding: Hebei University, 2012. [李泽健.低氧胁迫对中华绒螯蟹能量代谢、呼吸代谢及抗氧化代谢的影响[D].保定: 河北大学, 2012.]
|
| [9] |
Li T D, Brouwer M. Gene expression profile of grass shrimp Palaemonetes pugio exposed to chronic hypoxia[J]. Comparative Biochemistry and Physiology Part D:Genomics and Proteomics, 2009, 4(3): 196-208. DOI:10.1016/j.cbd.2009.03.004 |
| [10] |
Li T D, Brouwer M. Gene expression profile of hepatopancreas from grass shrimp Palaemonetes pugio exposed to cyclic hypoxia[J]. Comparative Biochemistry and Physiology Part D:Genomics and Proteomics, 2013, 8(1): 1-10. DOI:10.1016/j.cbd.2012.10.003 |
| [11] |
Martínez-Quintana J A, Peregrino-Uriarte A B, Gollas-Galván T, et al. The glucose transporter 1-GLUT1-from the white shrimp Litopenaeus vannamei is up-regulated during hypoxia[J]. Molecular Biology Reports, 2014, 41: 7785-7898. |
| [12] |
Li F H, Luan W, Zhang C S, et al. Cloning of cytoplasmic heat shock protein 90(FcHSP90) from Fenneropenaeus chinensis and its expression response to heat shock and hypoxia[J]. Cell Stress and Chaperones, 2009, 14(2): 161-172. |
| [13] |
Wei L, Qiu L G, Li Y H, et al. Comparision of the mitochondrial ultrastructure between two varieties of the shrimp Litopenaeus vannamei under hypoxia stress[J]. Journal of Tropical Biology, 2016, 7(1): 17-22. [魏琳, 邱立国, 李玉虎, 等. 低氧胁迫下不同品种凡纳滨对虾线粒体超微结构的比较[J]. 热带生物学报, 2016, 7(1): 17-22.] |
| [14] |
Fisheries and Fishery Administration, the Ministry of Agriculture. China Fishery Statistical Yearbook 2017[M]. Beijing: China Agriculture Press, 2017. [农业部渔业渔政管理局.中国渔业统计年鉴2017[M].北京: 中国农业出版社, 2017.]
|
| [15] |
Guan Y Q, Li L, Wang H C, et al. Effects of hypoxia on respiratory metabolism and antioxidant capability of Macrobrachium nipponense[J]. Journal of Hebei University:Natural Science Edition, 2010, 30(3): 301-306. [管越强, 李利, 王慧春, 等. 低氧胁迫对日本沼虾呼吸代谢和抗氧化能力的影响[J]. 河北大学学报:自然科学版, 2010, 30(3): 301-306.] |
| [16] |
Sun S M, Xuan F J, Fu H T, et al. Molecular characterization and mRNA expression of hypoxia inducible factor-1 and cognate inhibiting factor in Macrobrachium nipponense in response to hypoxia[J]. Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology, 2016, 196-97: 48-56. |
| [17] |
Sun S M, Ge X P, Fu H T, et al. Molecular cloning and expression analysis of the hemocyanin gene from oriental river prawn (Macrobrachium nipponense)[J]. Journal of Fishery Sciences of China, 2014, 21(2): 235-243. [孙盛明, 戈贤平, 傅洪拓, 等. 青虾血蓝蛋白基因的克隆、表达分析及多克隆抗体的制备[J]. 中国水产科学, 2014, 21(2): 235-243.] |
| [18] |
Sun S M, Ge X P, Fu H T, et al. Molecular cloning and gene expression of peroxiredoxin (Prx) in oriental river pawn (Macrobrachium nipponense) in response to environmental hypoxia and reoxygenation[J]. Journal of Fishery Sciences of China, 2014, 21(3): 474-483. [孙盛明, 戈贤平, 傅洪拓, 等. 日本沼虾过氧化物还原酶基因的克隆及其表达分析[J]. 中国水产科学, 2014, 21(3): 474-483.] |
| [19] |
Sun S M, Guo G B, Fu H T, et al. Based on the metabolomic approach the energy metabolism responses of oriental river prawn Macrobrachium nipponense hepatopancreas to acute hypoxia and reoxygenation[J]. Frontiers in Physiology, 2018, 9: 76. DOI:10.3389/fphys.2018.00076 |
| [20] |
Sun S M, Xuan F J, Ge X P, et al. Transciptomic and histological analysis of hepatopancreas, muscle and gill tissues of oriental river prawn (Macrobrachium nipponense) in response to chronic hypoxia[J]. BMC Genomics, 2015, 16: 491. DOI:10.1186/s12864-015-1701-3 |
| [21] |
Wu R S, Lam P K, Wan K L, et al. Tolerance to, and avoidance of, hypoxia by the penaeid shrimp (Metapenaeus ensis)[J]. Environmental Pollution, 2002, 118(3): 351-355. DOI:10.1016/S0269-7491(01)00298-6 |
| [22] |
Cheng Q, Chen X H, Luo Y J, et al. Preliminary study on asphyxiation point and oxygenation rate of Penaeus vannamei[J]. Journal of Hydroecology, 2001, 21(2): 14-15. [陈琴, 陈晓汉, 罗永巨, 等. 南美白对虾耗氧率和窒息点的初步测定[J]. 水生态学杂志, 2001, 21(2): 14-15.] |
| [23] |
Franco R, Sánchez-Olea R, Reyes E M, et al. Environmental toxicity, oxidative stress and apoptosis:Ménage à Trois[J]. Mutation Research, 2009, 674(1-2): 3-22. |
| [24] |
Wu Z H, You F, Wang Y F, et al. The effects of hypoxia and hyperoxia on nucleus anomaly, SOD, CAT activities and MDA content in juvenile turbot Scophthalmus maximus[J]. Journal of Shanghai Ocean University, 2011, 20(6): 808-813. [吴志昊, 尤锋, 王英芳, 等. 低氧和高氧对大菱鲆幼鱼红细胞核异常及氧化抗氧化平衡的影响[J]. 上海海洋大学学报, 2011, 20(6): 808-813.] |
| [25] |
Ren H, Li J, Li J T, et al. Effects of acute ammonia stresses on antioxidant enzyme activities and GPx gene expression in Exopalaemon carinicauda[J]. Journal of Agro-Environment Science, 2014, 33(4): 647-655. [任海, 李健, 李吉涛, 等. 急性氨氮胁迫对脊尾白虾(Exopalaemon carinicauda)抗氧化系统酶活力及GPx基因表达的影响[J]. 农业环境科学学报, 2014, 33(4): 647-655.] |
| [26] |
Zenteno-Savin T, Saldierna R, Ahuejote-Sandoval M, et al. Superoxide radical production in response to environmental hypoxia in cultured shrimp[J]. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 2006, 142(3-4): 301-308. |
| [27] |
Wang Q F, Shen W L, Hou C C, et al. Physiological responses and changes in gene expression in the large yellow croaker Larimichthys crocea following exposure to hypoxia[J]. Chemosphere, 2016, 169: 418-427. |
| [28] |
Li Y H. Studies on the growth and development rules and hypoxia stress of Litopenaeus vannamei[D]. Haikou: Hainan University, 2015. [李玉虎.凡纳滨对虾生长发育规律及对低氧胁迫响应的研究[D].海口: 海南大学, 2015.]
|
| [29] |
Chen S X. Golden pompano (trachinotus ovatus) liver, gill physiological and pathological changes and related gene expression under hypoxia stress[D]. Shanghai: Shanghai Ocean University, 2016. [陈世喜.卵形鲳鲹肝脏和鳃器官在急、慢性低氧胁迫下的生理组织及相关基因表达变化的研究[D].上海: 上海海洋大学, 2016.]
|
| [30] |
Li Y H. Oxidative stress, DNA damage and antioxidant enzyme activities in the Pacific white shrimp (Litopenaeus vannamei) when exposed to hypoxia and reoxygenation[J]. Chemosphere, 2016, 144: 234-240. DOI:10.1016/j.chemosphere.2015.08.051 |
| [31] |
Tao Y F, Qiang J, Wang H, et al. Acute toxicity of low-pH stress and its effect on enzyme activity and histological structure of gill and hepatopancreas in Procambarus clarkii[J]. Journal of Fishery Sciences of China, 2016, 23(6): 1279-1289. [陶易凡, 强俊, 王辉, 等. 低pH胁迫对克氏原螯虾鳃和肝胰腺酶活力及组织结构的影响[J]. 中国水产科学, 2016, 23(6): 1279-1289.] |
| [32] |
Wu X J. The effects of hypoxia on cell apoptosis and the activity of antioxidant enzymes megalobrama amblycephala[D]. Wuhan: Huazhong Agricultural University, 2015. [吴鑫杰.低氧对团头鲂细胞凋亡及抗氧化酶活性的影响[D].武汉: 华中农业大学, 2015.]
|
| [33] |
Sollid J, Nilsson G E. Plasticity of respiratory structures-Adaptive remodeling of fish gills induced by ambient oxygen and temperature[J]. Respiratory Physiology & Neurobiology, 2006, 154(1-2): 241-251. |
| [34] |
Matey V, Iftikar F I, De Boeck G, et al. Gill morphology and acute hypoxia:responses of mitochondria-rich, pavement, and mucous cells in the Amazonian oscar (Astronotus ocellatus) and the rainbow trout (Oncorhynchus mykiss), two species with very different approaches to the osmo-respiratory compromise[J]. Canadian Journal of Zoology, 2011, 89(4): 307-324. |
| [35] |
Sollid J, Kjernsli A, De Angelis P M, et al. Cell proliferation and gill morphology in anoxic crucian carp[J]. American Journal of Physiology:Regulatory, Integrative and Comparative Physiology, 2005, 289(4): R1196-R1201. DOI:10.1152/ajpregu.00267.2005 |
| [36] |
Matey V, Richards J G, Wang Y, et al. The effect of hypoxia on gill morphology and ionoregulatory status in the Lake Qinghai scaleless carp, Gymnocypris przewalskii[J]. Journal of Experimental Biology, 2008, 211(7): 1063-1074. DOI:10.1242/jeb.010181 |
| [37] |
Wang W N, Wang A L, Wei X H, et al. Studies on the ultrastructure of hepatopancreas cells of Macrobrachium nipponense[J]. Acta Zoologica Sinica, 1997, 43(S1): 39-44. [王维娜, 王安利, 魏渲辉, 等. 日本沼虾肝胰腺细胞超微结构的研究[J]. 动物学报, 1997, 43(S1): 39-44.] |
| [38] |
Han X L, Gao B Q, Wang H F, et al. Effect of low salinity on microstructure of gill and hepatopancreas and family survival rate of Portunus trituberculatus[J]. Progress in Fishery Science, 2014, 35(1): 104-110. [韩晓琳, 高保全, 王好锋, 等. 低盐胁迫对三疣梭子蟹鳃和肝胰腺显微结构及家系存活的影响[J]. 渔业科学进展, 2014, 35(1): 104-110. DOI:10.3969/j.issn.1000-7075.2014.01.015] |
| [39] |
Hong M L, Chen L Q, Gu S Z, et al. Effects of ammonia exposure on immunity indicators of haemolymph and histological structure of hepatopancreas in Chinese mitten crab (Eriocheir sinensis)[J]. Journal of Fishery Sciences of China, 2007, 14(3): 412-418. [洪美玲, 陈立侨, 顾顺樟, 等. 氨氮胁迫对中华绒螯蟹免疫指标及肝胰腺组织结构的影响[J]. 中国水产科学, 2007, 14(3): 412-418. DOI:10.3321/j.issn:1005-8737.2007.03.010] |
 2019, Vol. 26
2019, Vol. 26

