流行性溃疡综合征(epizootic ulcerative syndrome, EUS)是野生及咸淡水养殖鱼类头部或体表肌肉溃疡、骨骼肌形成典型霉菌性肉芽肿的一种季节性流行病。该病常发于冬末春初阴雨低温梅雨季节, 死亡率可高达100%[1]。1971年, 日本首次报道了这种疾病[2], 当时被称为霉菌性肉芽肿病(mycotic granulomatosis, MG)。随后澳洲[3-6]、北美[7-10]、东南亚[11-13]和南非[14]等国家相继多次报道。目前全球至少有26个国家发现该病, 可感染鱼类达100多种[15]。国际动物卫生组织(OIE)已确认侵袭丝囊霉菌(Aphanomyces invadans)是动物源性溃疡综合征(EUS)的病原菌体[16]。国内也曾有南方鲇[17]、鮸[18]、乌鳢[19]等疑似溃疡症状的报道, 但没有研究性文献报道丝囊霉菌病原与溃疡病相关。由于侵袭丝囊霉菌易在低温季节侵入表皮, 不易被觉察, 早期在肌肉中缓慢生长形成体表红斑, 逐步引起皮肤溃疡, 延伸至腹部、头盖骨等部位, 最终造成肌肉溃烂坏死, 在此过程中容易继发感染细菌、病毒而造成大量死亡, 因此易误诊。因丝囊霉菌对培养基要求较高且生长缓慢, 培养过程中易被细菌或其他真菌污染, 所以较难分离培养, 目前国内鲜见动物源丝囊霉菌病原的相关研究资料报道, 仍将其列为二类疫病[20]。
鳢(Channa maculata)属鲈形目(Perciformes)月鳢科(Channidae)鳢属(Ophiocephalus)。因其生长速度快, 抗病力强, 易饲养, 肉质鲜嫩, 蛋白质和人体必需的钙、磷、铁及多种维生素含量高, 且具有较高的药用价值, 是珠三角地区大规模养殖的淡水经济鱼类[21-22]。2012年以来, 冬末春初广东省养殖场鳢经常暴发体表溃疡, 躯干、背部和头盖骨形成大块溃烂或断尾的疾病, 发病率达80%, 死亡率达90%以上, 幸存者也因体表溃烂、丧失商品价值, 造成巨大经济损失。本研究利用形态学、组织学与分子生物学相结合的方法, 分离鉴定鳢源溃疡综合征丝囊霉菌病原, 对溃疡综合征的病原分离和鉴定进行了初步探索和基础性研究, 旨在为鱼类丝囊霉菌的病原学研究、流行病学调查、致病机理和疾病防治的深入研究奠定基础。
1 材料与方法 1.1 病鱼来源发病鱼样本采自珠三角某鳢养殖场, 体重300~500 g。发病水温15~20℃, 主要症状为体表溃疡(表 1)。
|
|
表 1 病鱼发病情况 Tab.1 Incidence of diseased fish |
取自然发病鱼的溃疡灶, 置于载玻片上, 活体压片, 同时进行Grocott六胺银染色[23], Nikon- e80显微镜观察和摄影。
将发病鱼溃疡灶, 用Bouin氏液固定24 h, 梯度酒精脱水, 二甲苯透明, 石蜡包埋。RM-2135型Leica轮转式切片机连续切片, 厚度为3~5 μm, HE常规染色和Grocott六胺银染色[23], Nikon-e80显微镜观察和摄影。
1.3 超微病理材料制备及扫描电子显微镜观察取发病鱼溃疡灶病变组织固定于2.5%戊二醛, 1%锇酸后固定, 系列乙醇脱水, 醋酸异戊酯置换, 常规临界干燥, 真空离子镀金, 在美国FEIQuanta250扫描电子显微镜观察、拍照。
1.4 病原分离与形态学鉴定参照行业标准[24], 从自然发病鱼溃疡灶处无菌切取2~4 mm2的小片肌肉, 置肉汤培养基, 25℃培养12 h, 将出现的菌丝转移到新鲜的琼脂平板上, 直到平板无其他污染菌为止。取带有生长活力菌丝置于肉汤培养基20℃孵育, 用灭菌的池塘水洗5次, 灭菌塘水中20℃过夜。大约12 h后观察原代孢囊群落的形成。Nikon倒置显微镜下观察, 显微摄影。
1.5 ITS序列分析 1.5.1 DNA的提取称取分离到的菌丝体50 mg, 液氮研成粉末转至1.5 mL的EP管内, 采用CTAB裂解法提取基因组DNA, 加600 μL缓冲液(2%CTAB; 1.4 mol/L NaCl; 0.2%β-巯基乙醇; 20 mmol/L EDTA; 100 mmol/L TrisHCl; pH8.0)充分混匀; 65℃水浴1 h, 每隔10 min振荡1次; 用等体积的Tris-饱和酚:氯仿:异戊醇(25:24:1)抽提1次, 再用氯仿:异戊醇(24:1)抽提1次; 取上清, 加入1.5倍体积的无水乙醇, 室温沉淀20 min, 4℃, 4000 r/min离心6 min, 收集沉淀, 作为PCR反应的模板。
1.5.2 ITS的PCR扩增及系统发育分析参考OIE手册霉菌特异性引物: F(5'-TCATTGTGAGTGAAACGGTG-3')和R1(5'-GGCTAAGGTTTCAGTATGTAG-3'), 目的条带有268 bp。20 μL PCR反应体系: 5×buffer 4 μL, 2.5 mmol/L dNTP 2 μL, 5 μmol/L上下游引物各1 μL, 0.4 μL TaqDNA聚合酶, DNA模板5 μL, 加入灭菌去离子水至20 μL。PCR反应程序为: 95℃, 3 min; 95℃, 30 s, 58℃, 30 s, 72℃, 30 s, 35个循环, 72℃延伸5 min。将PCR产物送生工生物工程公司测序。在GenBank中(http://www.ncbi.nlm.nih.gov)应用blastn对获得的基因片段进行同源性检索, 采用MEGA6.0软件构建系统进化树。
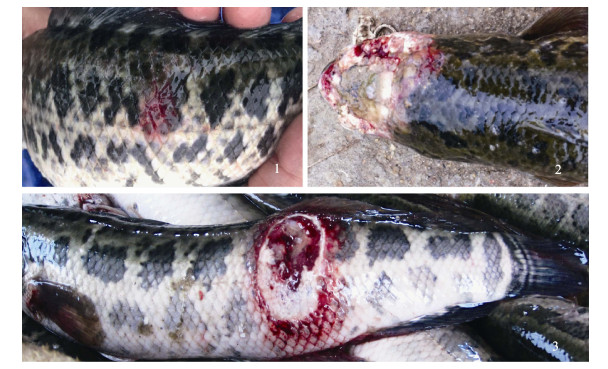
2 结果与分析 2.1 临床病变在低温潮湿季节, 养殖鱼多沉于塘底, 患病初期, 不易观察到病鱼。水温稍有回升时, 鱼开始上游, 可观察到部分鱼游动异常, 体表红斑(图 1-1); 随后病鱼体表红斑范围扩大, 逐步向躯干和头部延伸, 发展成体表溃疡, 溃疡灶周围充血红肿; 之后躯干体侧、背部和头部出现大面积溃烂(图 1-2, 图 1-3), 溃烂部位呈苍白色, 可闻到恶臭味; 严重溃烂病例头盖骨和肌肉溃烂坏死, 脑部和内脏裸露, 在疾病后期病灶表面出现肉眼可观察到白色丛生菌丝。
|
图 1 鳢流行性溃疡综合征外观 1:病发初期, 体表红斑; 2:头盖骨软组织和硬组织大面积溃烂坏死; 3:躯干皮肤和肌肉大块溃烂. Fig.1 Symptom of Epizootic Ulcerative Syndrome of Channa maculata 1: Red spot on the body surface of the initial stage of disease; 2: Large areas ulceration and necrosis on the soft and hard tissue of skull; 3: The mass ulceration on trunk skin and muscles. |
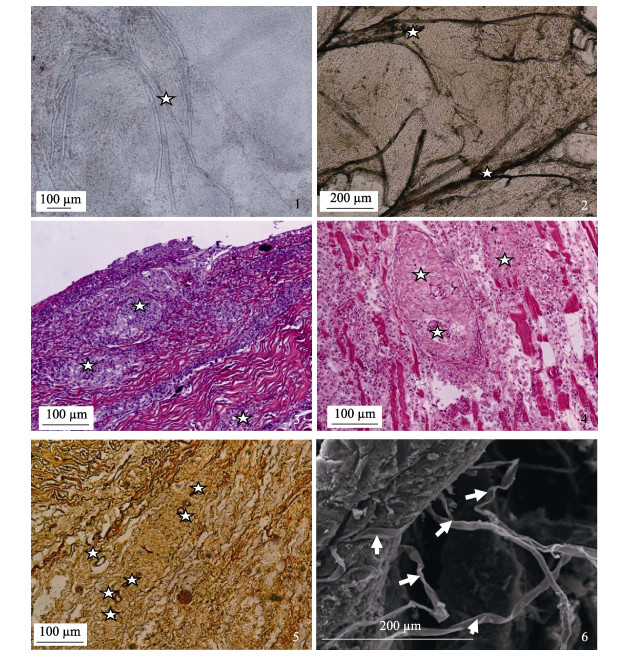
组织压片观察到溃疡肌肉组织中有大量丝状、纤细、很少分支的菌体(图 2-1), Grocott六胺银染色, 纤细菌丝呈棕黑色, 直径10~20 μm, 交错分布在肌肉中(图 2-2)。
|
图 2 溃疡组织病变及扫描电镜丝囊霉菌观察 1:活体压片组织中的真菌(☆); 2:组织中的真菌丝呈黑色或棕色(☆)Grocott六胺银染色; 3:侵袭到皮肤结缔组织中的霉菌形成的肉芽肿结节(☆), HE; 4:侵袭到肌肉组织中的霉菌形成的肉芽肿结节(☆), HE, ; 5:肌肉组织及肉芽肿结节中侵袭的真菌(☆), Grocott六胺银染色; 6:扫描电镜观察到溃疡肌肉中纤细的丝囊霉菌. Fig.2 Histopathological characteristics and Aphanomyces Invadans scanning by EMS of ulcer tissues 1: The Aphanomyces Invadans (star) of squashing tissue; 2: The Aphanomyces Invadans (star) of squashing tissue by Grocott hexamine silver stain; 3: The hyphae invading through the derma with granulomatous inflammation (star), HE staine; 4: The hyphae invading through the skeletal muscle with granulomatous inflammation (star), HE staine; 5: The hyphae (star) invading the derma, skeletal muscle and granulomatous inflammation, Grocott hexamine silver stain; 4: Ultramicrostructures of Aphanomyces Invadans by scanning electron microscope; 5: The hyphae (star) invading the derma, skeletal muscle and granulomatous inflammation, Grocott hexamine silver stain; 6: Ultramicrostructures of Aphanomyces Invadans by scanning electron microscope. |
病理组织切片HE染色, 光学显微镜观察到溃疡组织的皮肤致密结缔组织层(图 2-3)和肌肉层(图 2-4)有典型的霉菌肉芽肿结节。肉芽肿结节中央是真菌菌丝, 六胺银染呈褐色或棕色(图 2-5), 外围包绕着类上皮细胞和纤维细胞, 炎性细胞浸润在结节外层。轻微病例体表上皮细胞变性坏死, 脱落, 鳞片或真皮层裸露; 有些病例可观察到体表毛细血管扩张充血; 有些病例皮肤结缔组织层纤维溶解断裂, 有大量炎性细胞浸润。严重病例肌纤维水肿, 变性, 溶解, HE染色呈浅红色; 有些肌纤维凝固性坏死, 染色呈深红色(强嗜酸性); 有些肌纤维断裂溶解, 其间浸润大量的炎性细胞。扫描电镜观察到大量纤细的丝状真菌在肌肉组织中延伸, 菌丝表面较光滑、平整(图 2-6)。
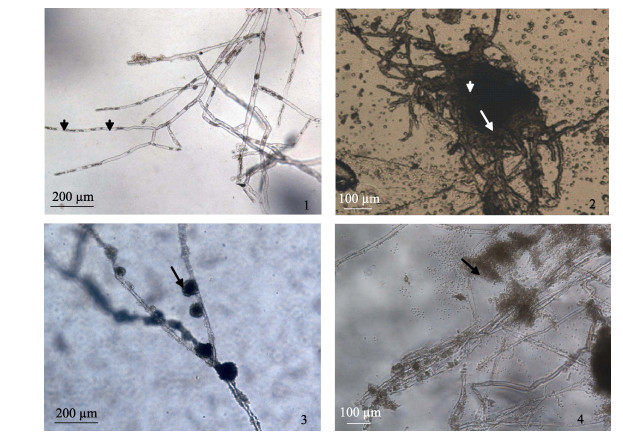
2.3 病原分离与形态学鉴定无菌操作下, 取2~3 mm3的溃烂病变肌肉组织于肉汤培养基培养12 h, 置显微镜下观察到直径10~20 μm纤细丝状菌丝, 菌丝分枝较少, 没有横膈膜, 孢子在菌丝中分段排列, 并有一定的间隔(图 3-1)。将出现的菌丝转移到新鲜的琼脂平板上纯化培养, 显微镜下观察菌丝菌落(图 3-2), 直至菌丝纯化为止, 获得HN 20170224和HN 20170314菌株。将菌丝置无菌塘水中20℃过夜, 镜下可观察到形成的原代孢子群(图 3-3)。有些动孢子释放出来, 不散开, 堆积在菌丝周围, 有些则分散在水中(图 3-4)。
|
图 3 病原霉菌形态观察 1:菌丝无横隔, 孢子在菌丝内分段排列; 2:固体培养基上的菌落; 3:原代孢囊; 4:释放出来的动孢子堆积在菌丝周围(箭头). Fig.3 Morphology of Aphanomyces Invadans 1: The fungus (star) of squashing tissue; 2: The filamentous hyphae were nonseptate with irregular walls and occasional branches, spore segmented arrangement in the hyphae (arrowhead); 2: The fungus on solid medium; 3: The original generation of cysts (arrow); 4: Released spores accumulation around the hyphae. |
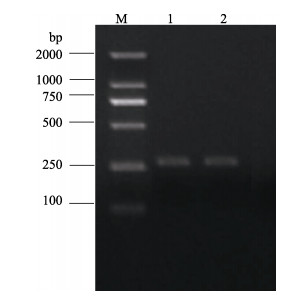
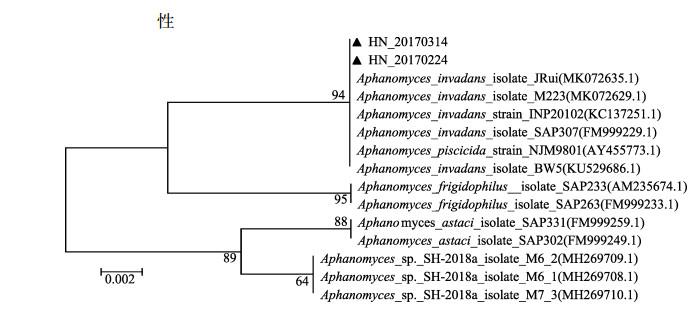
以HN 20170224和HN 20170314基因组DNA为模板, 采用霉菌特异性引物进行ITS的PCR扩增, 琼脂糖凝胶电泳后分别得到2条260 bp大小的清晰条带(图 4)。将扩增获得HN 20170224 (GenBank登录号为: MK855505)和HN 20170314 (GenBank登录号为: MK855505)分离株ITS序列在GenBank数据库中BLAST同源搜索, 选取13条相似性最高的菌种序列, 采用邻接法(Neighbor-Joining, NJ)构建系统发育树(图 5)。结果表明HN 20170224和HN 20170314与侵袭丝囊霉菌(Aphanomyces invadans) JRui、M223、INP20102、SAP307、BW5、杀鱼丝囊霉菌(Aphanomyces piscicida NJM9801, 与Aphanomyces invadans同物异名)[25]等归为一支, 亲缘关系最近, 一致性分析显示两者具有100%的序列相似性。
|
图 4 丝囊霉菌ITS的PCR扩增产物 M: DL2000分子量标准; 1 HN 20170224, 2 HN 20170314 Fig.4 The PCR amplification products of Aphanomyces invadans ITS M: DL2000 molecular weight marker; 1 HN 20170224, 2 HN 20170314 |
|
图 5 菌株HN 20170224的ITS基因序列构建的系统进化树 Fig.5 Phylogenetic tree based on ITS gene sequence of the strain HN 20170224 |
引起鱼类溃疡病的病原报道较多, 但目前国际上大部分学者认为侵袭丝囊霉菌是引起鱼类流行性溃疡综合征的主要病原[2, 9, 17, 22], 其他病原也和本病流行有关, 可成为EUS的继发感染病原。John等[26]报道鳢科鱼类溃疡综合征可分离出弹状病毒和双RNA病毒。Callinan等[5]在红斑病(RSD)鲻中分离到气单胞菌属(Aeromonas spp.), 产碱杆菌属(Alcaligenes spp.), 假单胞菌(Pseudomonas spp.)和弧菌(Vibrio spp.), 但最终都经试验证实这些病原无法直接造成溃疡综合征。Kiryu等[27]报道丝囊霉菌游动孢子感染大西洋油鲱22 d后可形成霉菌性肉芽肿, 并引起肌肉溃烂。国内也有大量资料报道嗜水气单胞菌、温和气单胞菌等条件致病菌可引起溃疡综合征。吉莉莉等[17]等从南方鲇溃疡综合征中分离出豚鼠气单胞菌; 胡健饶等[18]在鮸鱼溃疡病种分离出美人鱼发光杆菌; 史秀杰等[28]从溃疡北极红点鲑进行真菌分离, 未进行形态鉴别, 分子生物方法鉴定为水霉菌, 未能证实丝囊霉菌感染引起的溃疡。殷中琼等[19]对乌鳢体表溃疡进行过病理学研究, 临床症状与本病相似, 但病理变化未报到有肉芽肿结节, 也未进行真菌分离, 仅提到多种抗生素治疗无效, 提示可能是其它病原引起。
肖秀娥等[29]2009年在非洲南部的赞比西河谷地区进行过EUS的流行性调查, 并依据当地调查结果提出应在国内引起足够重视和科学研究的建议。彭开松等[30]对溃疡综合征进行了综述, 国内目前未查到关于溃疡病灶丝囊霉菌分离和致病性研究的相关资料报道, 病原特性、致病情况、快速检测方法和免疫防治等方面的研究工作还未开展起来。本研究在溃疡灶处分离出真菌, 从形态学和孢子囊的培养特性、ITS的PCR扩增结果分析和病理组织学特征入手, 在国内率先证实分离真菌为侵袭丝囊霉菌。观察到大部分体侧、背部、尾部和头部溃疡特征与流行性溃疡综合征临床症状相似[2, 4, 10, 16]。溃烂组织活体压片和石蜡切片HE染色及Grocott六胺银染观察到分布在坏死的肌肉中大量纤细的丝状霉菌和典型的霉菌结节, 与资料报道一致[10, 11, 27]。诱导无性生殖结构(孢子)可用于丝囊霉菌属的鉴定, 分离培养可观察到长丝状, 无横隔分支少的丝状霉菌, 纯化的真菌丝在无菌池水20℃过夜, 大约12 h后形成原代孢囊群落, 霉菌形态与原代孢囊与行业标准检测基本一致[24]。扫描电镜观察到菌丝细长, 从肌肉组织中延伸出来。ITS的PCR扩增结果分析与侵袭丝囊菌(A. invadans)相似率达100%。依据临床症状, 在溃疡鳢中分离出真菌病原形态、原代孢囊群落形成特征、典型真菌肉芽肿结节和分子生物学鉴定为侵袭丝囊菌; 为确定丝囊霉菌是否为溃疡病的病原问题, 近7–8年, 研究者对大量发病鳢进行病原分离, 在低温季节不同地方爆发的溃疡病鱼100%都可以从组织中观察分离到丝囊霉菌, 但每次分离出细菌病原各不相同, 因此诊断为丝囊霉菌引起的流行性溃疡疾病。
研究者使用丝囊霉菌菌丝人工感染健康鳢20 d可以复制出体表红斑症状, 压片可以观察到丝状霉菌, 但结果不理想, 因该病的发生对环境和温度条件要求较高, 所以症状复制成功率低, 关于攻毒的方式和方法有待进一步摸索和提高。
该病目前已在珠江三角洲地区流行多年, 近二三年的多雨低温季节持续发病率很高, 病程可以延长到10月份, 已对南方鳢的养殖造成严重的影响。我们分离鉴定了鳢源侵袭丝囊霉菌病原, 积累了丝囊霉菌分离的经验, 为鱼类丝囊霉菌的病原学研究、致病机理、感染机制和免疫防治的深入研究提供了可靠的基础资料, 这也是今后重点研究的方向。
| [1] |
Iberahim N A, Trusch F, van West P. Aphanomyces invadans, the causal agent of epizootic ulcerative syndrome, is a global threat to wild and farmed fish[J]. Fungal Biology Reviews, 2018, 32: 118-130. DOI:10.1016/j.fbr.2018.05.002 |
| [2] |
Egusa S, Masuda N. A new fungal of Plecoglossus altivelis[J]. Fish Pathology, 1971, 6(1): 41-46. DOI:10.3147/jsfp.6.41 |
| [3] |
McKenzie R A, Hall W T K. Dermal ulceration of mullet (Mugil cephalus)[J]. Australian Veterinary Journal, 1976, 52(5): 230-231. DOI:10.1111/j.1751-0813.1976.tb00076.x |
| [4] |
Haines A K. Fish Fauna and Ecology, the Purari:Tropical Environment of a High Rainfall River Basin[M]. Hague: Dr W. Junk Publishers, 1983: 367-384.
|
| [5] |
Callinan R B, Keep J A. Bacteriology and parasitology of red spot disease in sea mullet, Mugil cephalus L., from eastern Australia[J]. Journal of Fish Diseases, 1989, 12(4): 349-356. DOI:10.1111/j.1365-2761.1989.tb00323.x |
| [6] |
Dykstra M J, Noga E J, Levine J F, et al. Characterization of the Aphanomyces species involved with ulcerative mycosis (UM) in menhaden[J]. Mycologia, 1986, 78(4): 664-672. DOI:10.1080/00275514.1986.12025303 |
| [7] |
Hargis J R, William J. Quantitative effects of marine diseases on fish and shellfish populations[C]//Transaction North America Wildlife Naturalis Resource Conference, 1985, 50: 608-640.
|
| [8] |
Dykstra M J, Levine J F, Noga E J, et al. Ulcerative mycosis:a serious menhaden disease of the southeastern coastal fisheries of the United States[J]. Journal of Fish Diseases, 1989, 12(2): 175-178. DOI:10.1111/j.1365-2761.1989.tb00289.x |
| [9] |
Blazer V S, Lilley J H, Schill W B, et al. Aphanomyces invadans in Atlantic menhaden along the east coast of the United States[J]. Journal of Aquatic Animal Health, 2002, 14(1): 1-10. DOI:10.1577/1548-8667(2002)014<0001:AIIAMA>2.0.CO;2 |
| [10] |
Saylor R K, Miller D L, Vandersea M W, et al. Epizootic ulcerative syndrome caused by Aphanomyces invadansin captive bullseye snakehead Channa marulius collected from south Florida, USA[J]. Diseases of Aquatic Organisms, 2010, 88: 169-175. DOI:10.3354/dao02158 |
| [11] |
Lilley J H, Callinan R B, Chinabut S, et al. Epizootic Ulcerative Syndrome (EUS) Technical Handbook[M]. Bangkok: Quatic Animal Health Research Institute, 1998.
|
| [12] |
Roberts R J, Willoughby L G, Chinabut S. Mycotic aspects of epizootic ulcerative syndrome (EUS) of Asian fishes[J]. Journal of Fish Diseases, 1993, 16(3): 169-183. DOI:10.1111/j.1365-2761.1993.tb01248.x |
| [13] |
Vishwanath T S, Mohan C V, Shankar K M. Epizootic ulcerative syndrome (EUS), associated with a fungal pathogen, in Indian fishes:histopathology-'a cause for invasiveness'[J]. Aquaculture, 1998, 165(1-2): 1-9. DOI:10.1016/S0044-8486(98)00227-0 |
| [14] |
Andrew T G, Huchzermeyer K D A, Mbeha B C, et al. Epizootic ulcerative syndrome affecting fish in the Zambezi River system in Southern Africa[J]. Veterinary Record, 2008, 163(21): 629-631. DOI:10.1136/vr.163.21.629 |
| [15] |
Kamilya D, Baruah A. Epizootic ulcerative syndrome (EUS) in fish:history and current status of understanding[J]. Reviews in Fish Biology and Fisheries, 2014, 24(1): 369-380. DOI:10.1007/s11160-013-9335-5 |
| [16] |
O IE. Manual of Diagnostic Tests for aquatic animals[M]. Paris: World Organization for Animal Health, 2013: 1-13.
|
| [17] |
Ji L L, Wang K Y, Xiao D, et al. Isolation and identification of pathogenic bacteria causing ulcer disease of Silurusmeriordinalis Chen[J]. Freshwater Fisheries, 2008, 38(3): 68-72. [吉莉莉, 汪开毓, 肖丹, 等. 南方鲇溃疡综合征病原菌的分离与鉴定[J]. 淡水渔业, 2008, 38(3): 68-72. DOI:10.3969/j.issn.1000-6907.2008.03.013] |
| [18] |
Hu J R, Zhang L M, Shi Y H, et al. Isolation and identification of a pathogenic bacterium causing ulcer disease of miiuy croaker (Miichthys miiuy)[J]. Journal of Hangzhou Normal University (Natural Science Edition), 2011, 10(5): 444-465. [胡健饶, 章丽梅, 史雨红, 等. 一株鮸鱼溃疡致病菌的分离及鉴定[J]. 杭州师范大学学报(自然科学版), 2011, 10(5): 444-465. DOI:10.3969/j.issn.1674-232X.2011.05.011] |
| [19] |
Yin Z Q, Jia R Y, Tang F S. Study on pathomorphology of UDS in snakehead[J]. Sichuan Journal of Animal Husbandry and Veterinary Medicine, 1999, 13(3): 27-31. [殷中琼, 贾仁勇, 唐发书. 乌鳢溃疡性疾病综合征(UDS)的病理形态学研究[J]. 四川畜牧兽医学报, 1999, 13(3): 27-31.] |
| [20] |
Ministry of Agriculture of the People's Republic of China. A catalogue of animal epidemics of the first, second and third classe[R]. Announcement of Ministry of Agriculture 200811125. [中华人民共和国农业部.一二三类动物疫病病种名录[R].农业部公告[2008]11125号.]
|
| [21] |
Liu W K, Fan Q X, Zhu B K, et al. Effects of prey density on the growth and survival of larval hybrid snakehead[J]. Journal of Huazhong Agricultural University, 2007, 26(3): 367-370. [刘文奎, 樊启学, 朱邦科, 等. 饵料密度对杂交鳢仔鱼生长、存活的影响[J]. 华中农业大学学报, 2007, 26(3): 367-370. DOI:10.3321/j.issn:1000-2421.2007.03.022] |
| [22] |
Zou L G, Feng X Y, Wang Y X, et al. Comparative study on flesh quality of hybrid snakehead (Channa maculate ♀×Channa argus ♂) and Channa argus[J]. Journal of Shanghai Ocean University, 2011, 20(2): 303-307. [邹礼根, 冯晓宇, 王宇希, 等. 杂交鳢(斑鳢♀×乌鳢♂)与乌鳢肌肉品质比较研究[J]. 上海海洋大学学报, 2011, 20(2): 303-307.] |
| [23] |
Wang B Y, Li Y S, Huang G S, et al. Pathology Technology[M]. Beijing: People's Medical Publishing House, 2000: 160. [王伯云, 李玉松, 黄高昇, 等. 病理学技术[M]. 北京: 人民卫生出版社, 2000: 160.]
|
| [24] |
General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China. Trade Standard of Entry and Exit Inspection and Quarantine of the People's Republic of China, SN-T2120-2008[S]. [中华人民共和国国家质量检验检疫局.中华人民共和国出入境检验检疫行业标准, SN-T2120-2008[S].]
|
| [25] |
Makkonen J, Vesterbacka A, Martin F, et al. Mitochondrial genomes and comparative genomics of Aphanomyces astaci and Aphanomyces invadans[J]. Scientific Reports, 2016, 6: 36089. DOI:10.1038/srep36089 |
| [26] |
John K R, George M R. Viruses associated with epizootic ulcerative syndrome:an update[J]. Indian Journal of Virology, 2012, 23(2): 106-113. DOI:10.1007/s13337-012-0108-x |
| [27] |
Kiryu Y, Shields J D, Vogelbein W K, et al. Infectivity and pathogenicity of the oomycete Aphanomyces invadans in Atlantic menhaden Brevoortia tyrannus[J]. Diseases of Aquatic Organisms, 2003, 54: 135-146. DOI:10.3354/dao054135 |
| [28] |
Shi X J, Liu H, Gao L Y, et al. Isolation and identification of causative agents of sick Arctic charr (Salvelinus alpinus)[J]. Journal of Huazhong Agricultural University, 2007, 26(2): 223-227. [史秀杰, 刘荭, 高隆英, 等. 患病北极红点鲑的病原分离与鉴定[J]. 华中农业大学学报, 2007, 26(2): 223-227. DOI:10.3321/j.issn:1000-2421.2007.02.019] |
| [29] |
Xiao X E, Alushe H, Tjihuiko U. Investigation of the epizootic ulcerative syndrome (EUS) and its revelation[J]. China Animal Quarantine, 2010, 27(3): 52-53. [肖秀娥, Alushe H, Unda T. 动物流行性溃疡综合征(EUS)的调查及启示[J]. 中国动物检疫, 2010, 27(3): 52-53. DOI:10.3969/j.issn.1005-944X.2010.03.022] |
| [30] |
Peng K S, Fan H M, Ma Q Q, et al. Research progress in epizootic granulomatous aphanomycosis[J]. Journal of Anhui Agricultural University, 2018, 45(1): 25-29. [彭开松, 樊慧敏, 马倩倩, 等. 流行性肉芽肿性丝囊霉菌病研究进展[J]. 安徽农业大学学报, 2018, 45(1): 25-29.] |
 2019, Vol. 26
2019, Vol. 26

